Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
Breaking Boundaries: Novel Effects of Levosimendan in Various Diseases
Hongyuan Zhang 1, Minxing Zhao 2, and Yanrong Liu 1,3,*
1 Michael Smith building, Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, M139PT Manchester, UK.
2 Loreto High School, Chorlt on, M217SW Manchester, UK.
3 The Department of Cardiology, the 1st Affiliated Nanjing Medical University, 300 Guangzhou Road, Nanjing, China.
* Correspondence: yanrong.liu@manchester.ac.uk
Received: 6 February 2024
Accepted: 23 February 2024
Published: 18 March 2024
Abstract: Levosimendan, an inodilator that has been applied in clinical use for over two decades, has transcended its initial indication in the management of acutely decompensated chronic heart failure. Over the years, it has been adopted in septic shock, perioperative use of cardiac surgery, advanced end-stage heart failure, and has shown potential for inhaled administration, highlighting its versatility. Levosimendan has diverse mechanisms of action which mediate its non-traditional uses. Ongoing research aims to expand our understanding and develop personalized treatment strategies for the use of levosimendan. The significance of levosimendan in acute decompensated heart failure and cardiogenic shock, highlights its evolving role in contemporary cardiovascular medicine. This comprehensive review explores its pharmacodynamics, effects, and the challenges and opportunities it presents in various clinical settings. We describe levosimedan’s expanding usage, ranging from septic shock, intermittent intravenous in advanced heart failure, perioperative cardiac surgery and pulmonary hypertension management by inhaled levosimendan as well as its future prospects.
Keywords:
Levosimendan septic shock cardiac surgery advanced heart failure1. Introduction
Levosimendan was first used in routine clinical practice in 2000 for the management of decompensated severe chronic heart failure. Since then it has become an important player in cardiovascular pharmacology. This first-in-class inodilator, acting through a tripartite mechanism involving calcium sensitization, vasodilation, and cytoprotection, has attracted significant attention for its diverse effects [1]. Unlike traditional inotropic agents, levosimendan enhances cardiac contractility without increasing myocardial oxygen consumption, suggesting it to be a promising option for several cardiovascular conditions [2].
Over the past two decades, levosimendan has been used for conditions beyond its initial indications, with ongoing researches uncovering its potential benefits in diverse clinical scenarios. Levosimendan transcends its established role in acute heart failure treatment to cover several conditions such as septic shock and perioperative cardiac surgery [3]. This versatility makes it ideal for personalized treatment of various cardiovascular disorders. This review describes levosimendan’s mechanisms, clinical applications, and its potential to reshape contemporary cardiovascular medicine. The expanding scope of levosimendan including the treatment of acute decompensated heart failure and cardiogenic shock suggests that this drug is expected to revolutionize cardiovascular pharmacology. The is a narrative review that covers extensive literature but no meta-analysis is conducted in this article.
2. Pharmacodynamics of Levosimendan
Levosimendan is a first-in-class drug acts as an inodilator through a tripartite mechanism which involves acting as calcium sensitizer by increasing the sensitivity of troponin C fibers to ionic calcium and as a vasodilator and cytoprotective agent through opening of potassium channel on vascular smooth muscle cells and in mitochondria. It has gained significant attention in cardiovascular pharmacology. Levosimendan has one active metabolite, coded OR-1896 (see Figure 1) [4]. Both the parent drug and OR-1896 have similar effects, but levosimendan has a half-life of about 1h, whereas OR-1896 reaches its peak plasma concentration 2-3 days after levosimendan infusion, thus prolonging therapeutic effects beyond the infusion period. The drug was formulated for a 24-hour infusion, after which its pharmacodynamic effects persist for at least one week [5].
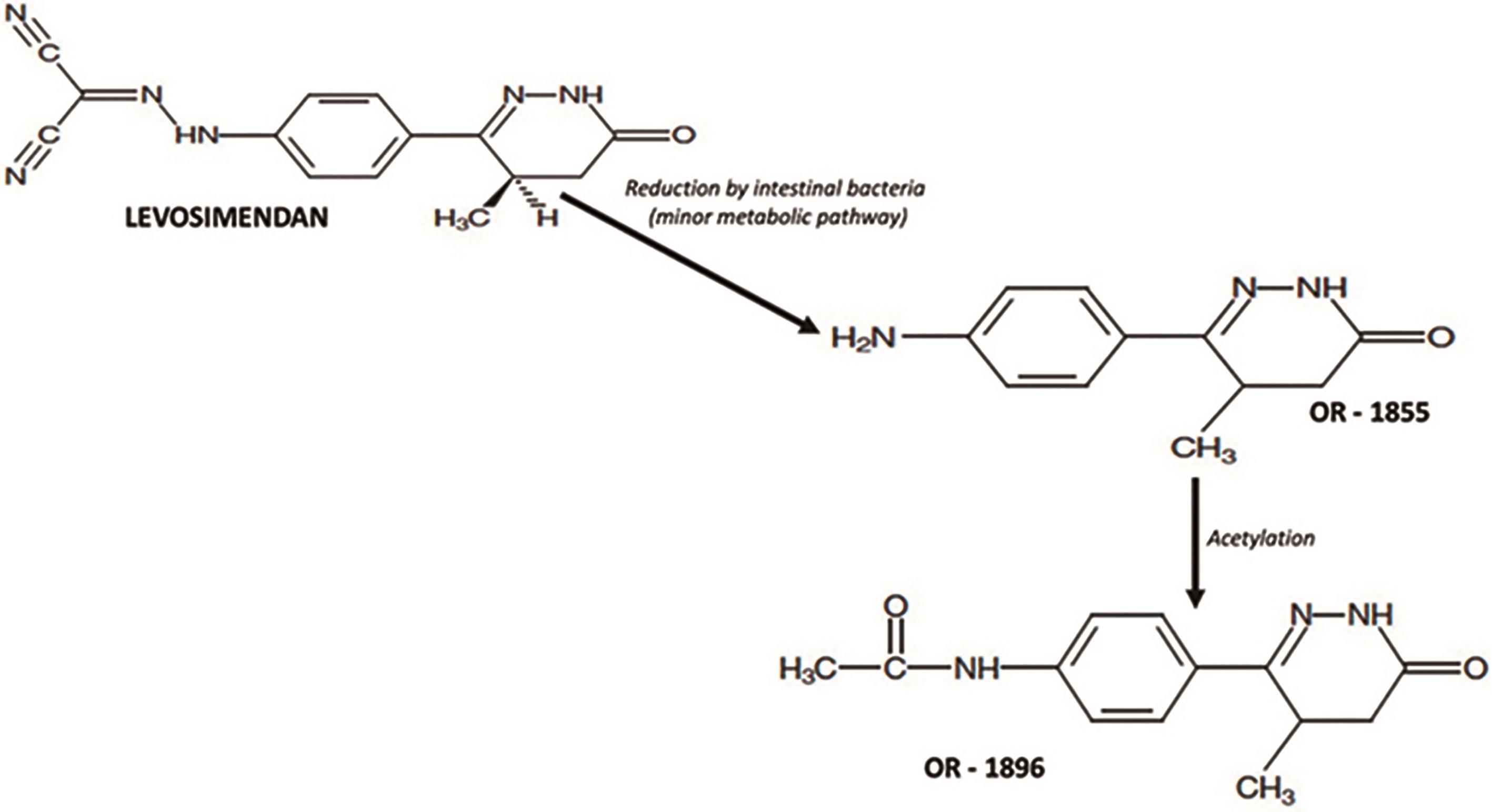
Figure 1. Metabolic pathway of transformation of levosimedan in its active metabolic.
3. The Effects of Levosimendan
Levosimendan can alleviate heart failure symptoms and reduce mortality in several clinical conditions. Its therapeutic effects extend beyond improving cardiac functionto include neurohormonal and anti-inflammatory effects. Notably, it has been shown to decrease brain natriuretic peptide, a key biomarker of heart failure severity, and pro-inflammatory cytokines like tumor necrosis factor-alpha and interleukin-6. In addition, it improves the right ventricular function and endothelial function as well as modulate coronary blood flow [6].
Besides its “traditional” indications, levosimendan may be used in septic shock, perioperative of cardiac surgery, and right heart failure due to pulmonary hypertension. Traditionally, levosimendan was mainly applied as a continuous intravenous administration. However, innovative strategies are emerging for its use in managing advanced heart failure, which include intermittent dosing and inhaled levosimendan in the treatment of pulmonary vasodilation. However, these approaches have not been incorporated in the current guidelines due to the limited evidence. Thus, physician discretion can be exercised in selecting levosimendan for these specific clinical situations. In this review, we explore the details of these innovative applications of levosimendan.
4. Levosimendan in Septic Shock and Septic Cardiomyopathy
Sepsis is characterized by an infection triggering dysfunction in at least one organ owing to a deregulated host inflammatory response. Septic shock is specifically defined as sepsis with persistent hypotension requiring vasopressors to maintain a mean arterial pressure ≥ 65 mmHg and a serum lactate level > 2 mmol/l despite adequate fluid challenge [7]. Septic shock can be fatal owing to the enhanced vascular hypo-reactivity and autonomic dysfunction. It can also induce septic cardiomyopathy with de novo acute heart failure contributing a sepsis mortality of about 30% [8]. Effective management of septic cardiomyopathy revolves around addressing the underlying infection and implementing appropriate fluid management strategies [9]. In cases of septic shock where patients are unresponsive or display inadequate responses to fluid resuscitation, noradrenaline is administered as the preferred vasopressor. Its administration can achieve the target mean arterial pressure of > 65 mmHg, and thus mitigate the severe hemodynamic consequences associated with septic shock [10].
Among the available clinical trials, the LeoPARDS trial (Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis), which investigated the use of levosimendan in septic shock suggested that levosimendan enhanced cardiovascular function in septic patients without significantly increasing myocardial oxygen consumption [11]. Another particular trial (Levosimendan versus dobutamine in myocardial injury patients with septic shock) evaluated the use of levosimendan in comparison to dobutamine in patients with septic shock, which demonstrated levosimendan reduced biomarkers of myocardial injury and improved hemodynamics compared with dobutamine [12]. Pioneer studies showed a promising outlook regarding the application of levosimendan in septic shock, with many trials reporting favorable outcomes such as improved cardiac function (higher stroke volumes and cardiac indices) and reduced biomarkers of myocardial injury compared to dobutamine. However, larger meta-analyses, e of Ge et al., did not find a statistically significant association between levosimendan and reductions in 28-day mortality or SOFA scores, despite improvements in cardiac parameters and serum lactate levels (see Figure 2). This seemingly divergent scenario might reflect the complex nature of septic shock, a multifaceted stress response, where targeting a single factor like cardiac function may not directly translate to improved mortality [13].
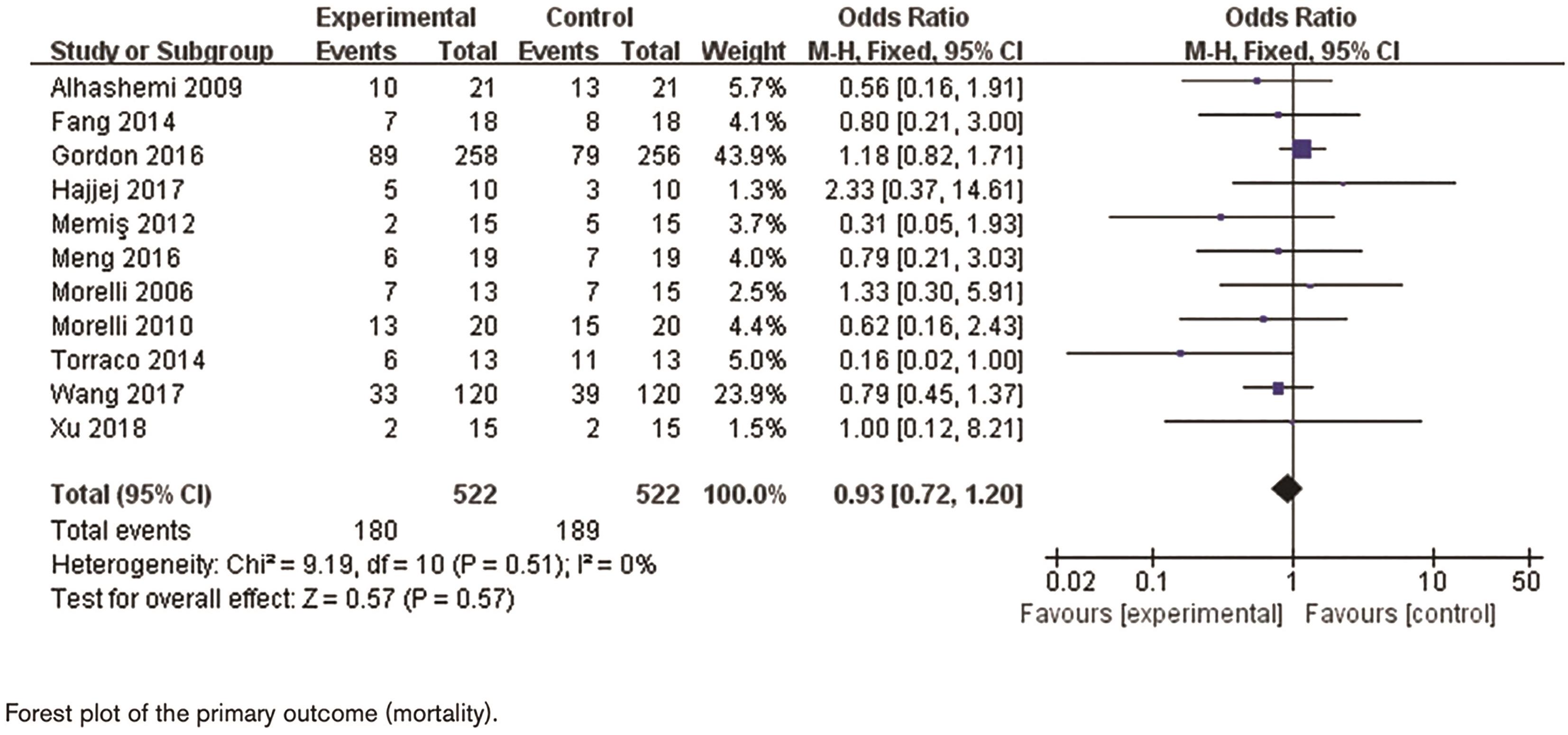
Figure 2. The meta-analysis of levosimendan in patients with septic shock.
Levosimendan’s unique ability to influence both myocardial and vascular function makes it a promising candidate for septic shock management, where maintaining a balance between these systems is crucial. In our clinical practice, we reserve levosimendan for patients exhibiting moderate to severe left ventricular systolic dysfunction and impaired end-organ perfusion on echocardiography. Future research should refine the roles of levosimendan by examining the optimal timing and dosage based on the severity of acute heart failure and aiming to maximize its cardioprotective benefits. While concerns regarding potential increases in vasopressor requirements exist, these may be outweighed by the broader benefits of levosimendan.
5. Perioperative Use in Cardiac Surgery
Levosimendan has gained significant attention for its potential to improve perioperative outcomes, particularly in cardiac surgery. Several investigations have been conducted to explore the role of levosimendan in perioperative cardiac surgery. It has been shown to augment contractility without increasing oxygen demand demonstrating its potential advantages, including reduced mortality and morbidity [14].
In the context of cardiac surgery, levosimendan can improve patient symptoms during the perioperative period. Unlike traditional inotropic agents, which increase myocardial oxygen demand and elevate risks in patients with limited cardiac reserve, levosimendan, amplifies cardiac contractility without significantly raising oxygen consumption. Clinical studies suggest that levosimendan can diminish mortality and morbidity in cardiac surgery patients, presenting itself as a valuable supplement to traditional inotropic support.
Levosimendan’s impact on perioperative outcomes extends beyond cardiac surgery, offering benefits that optimize patient care. This includes reducing perioperative myocardial injury, stabilizing hemodynamics, and minimizing the need for additional inotropic agents. Levosimendan’s unique dual actions, enhancing myocardial contractility and inducing vasodilation, improve cardiac performance throughout the perioperative period. Furthermore, its potential to reduce postoperative complications, including arrhythmias and organ dysfunction, positions levosimendan as a valuable tool for enhancing perioperative care and improving outcomes in cardiac surgery patients [15].
Several trials, including the CHEETAH Trial [16] and LEVO-CTS Trail [17], have explored the benefits of levosimendan in cardiac surgery. These trials suggest that levosimendan may decrease the occurrence of low cardiac output syndrome and maintain hemodynamic stability in patients undergoing cardiac surgery. Its capacity to improve cardiac contractility without elevating oxygen demand makes it ideal for this clinical setting [18].
Furthermore, clinical trials have investigated levosimendan’s role in preventing or managing postoperative complications, including acute kidney damage and atrial fibrillation. Some of studies proposed that it exerts a renoprotectiveeffect and decreases the incidence of atrial fibrillation in patients compared with conventional treatments [19].
In summary, evidence suggests that levosimendan has a positive impact on cardiac function, hemodynamic stability, and potential benefits in averting postoperative complications. Nevertheless, further research is needed to support the use of levosimendan in perioperative care of patients undergoing cardiac surgery.
6. Repetitive Use of Levosimendan in the Management of Advanced Heart Failure
Patients with advanced heart failure (AdHF) have high levels of mortality and morbidity. They also manifest with severe progression of symptoms and frequent rehospitalizations [20]. Despite the availability of guideline-based therapies, including diuretics, angiotensin-receptor/neprilysin inhibitors, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, mineralocorticoid receptor antagonists, beta-adrenergic blockers, and SGLT2 inhibitors, the use of conventional adrenergic inotropes has been associated with increased mortality rates.
To achieve long-term symptomatic relief and palliation, inodilators have been suggested, with levosimendan emerging as a prominent choice. Given its unique pharmacokinetics, levosimendan allows for intermittent dosing, making it an ideal drug for the management of these patients [21]. The intermittent dosing strategy of levosimendan can alleviate symptoms commonly associated with end-stage heart failure, such as dyspnea and fatigue. This approach is particularly beneficial for patients with advanced heart failure who may not tolerate the continuous administration of inotropic agents because it minimizes the risk of adverse effects observed with continuous infusions. Understanding how levosimendan enhances functional capacity and quality of life in outpatient settings will allow the establishment of approaches for the management of heart failure.
Several small-scale, non-randomized trials and registries involving advanced heart failure with reduced ejection fraction (HFrEF) patients (see Table 1), who are either ineligible or awaiting a heart transplant or LVAD implant, have demonstrated that repeated infusions of levosimendan improves outcomes of these patients. Intermittent infusion of levosimendan has been shown to ameliorate symptoms, clinical and hemodynamic status, prevent hospitalization due to recurrent heart failure, and optimize guideline-directed medical therapy. These evidence supports the recommendation of levosimendan as a valuable intervention in the comprehensive management of advanced heart failure patients.
Table 1. Repetitive levosimendan infusion for patients with advanced chronic heart failure.
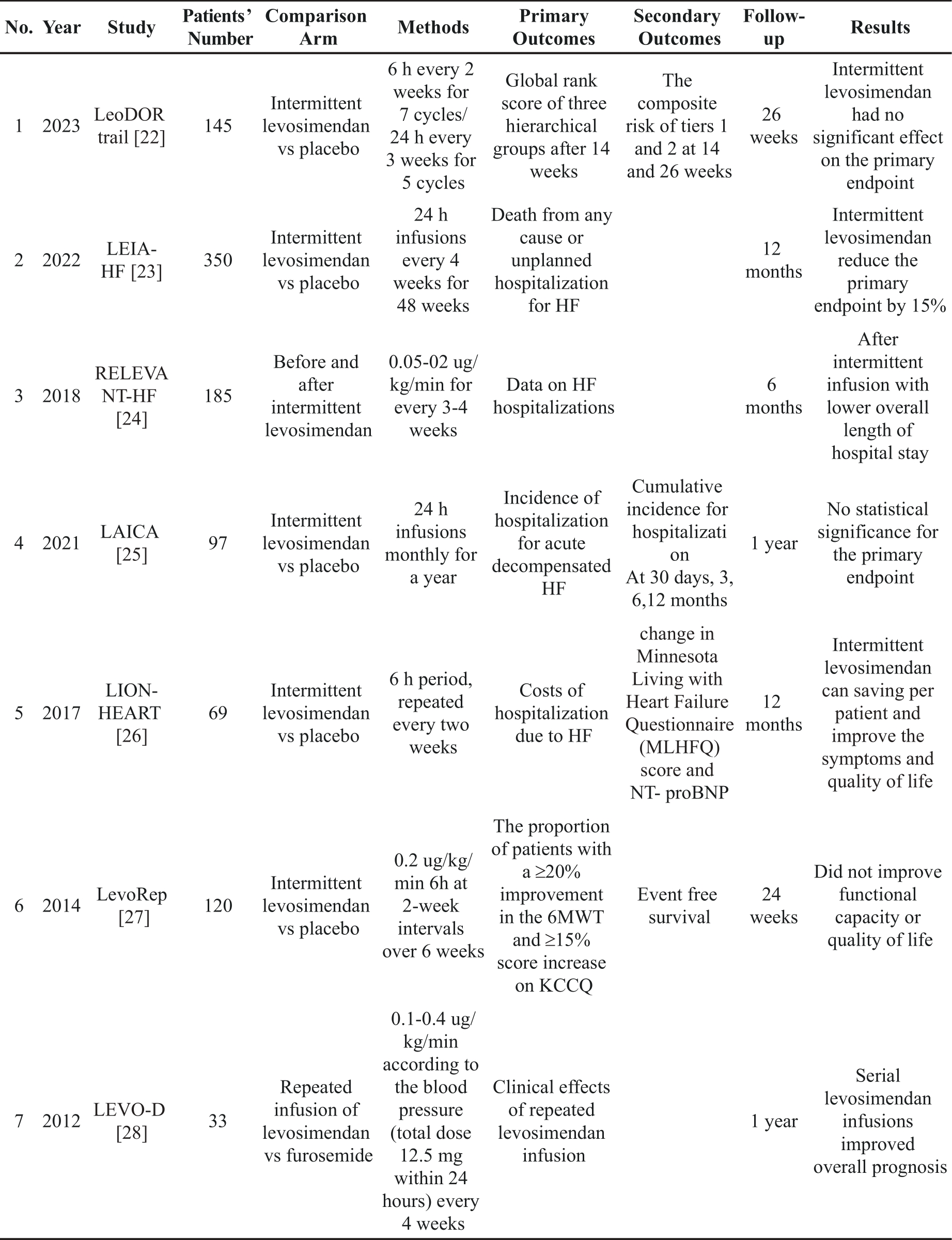
Clinical trials investigating the repetitive use of levosimendan in patients with advanced heart failure with reduced ejection fraction have been conducted in recent years [29]. Although the study groups tended to show similar outcomes in terms of clinical status, endpoints were different in each trial. While the majority of trials have shown significant benefits for patients receiving levosimendan, past experiences highlight challenges in using symptoms as an objective endpoint in heart failure trials. Symptoms may not be consistently measurable and may not serve as an ideal primary endpoint for testing treatment efficacy. All previous clinical trials revealed that repetitive levosimendan infusions are safe and multiple infusions of levosimendan have several benefits. The use of levosimendan has shifted from the management of acute heart failure to a safe and potentially effective treatment for outpatients with advanced HFrEF [30].
The most recent meta-analysis, conducted by Slivetti Simona et al and published in 2024, meticulously selected 9 trials characterized by specific attributes suitable for a meta-analysis on mortality. The findings underscore the association of repetitive/intermittent therapy with i.v. levosimendan with a noteworthy reduction in mortality at the longest available time point: 50 out of 399 (12.5%) compared with 60 out of 281 (21.4%) (95% confidence interval, 0.42-0.90, P < 0.01). It is imperative to acknowledge, however, the presence of several limitations in this meta-analysis, including a heterogeneous population, varied selection of comparators, diverse time points of collecting mortality data, and the inclusion of nonblinded studies. Notwithstanding these methodological constraints, the study advocates for a continued and nuanced exploration of the repetitive use of levosimendan in patients with AdHF through meticulously designed, adequately powered regulatory clinical trials. Future investigations should not only prioritize the reduction of long-term mortality but also encompass a comprehensive evaluation of quality-of-life parameters, encompassing symptom relief and the mitigation of rehospitalization risks. Additional clinical data are required to substantiate the multidirectional effects of levosimendan in patients with heart failure [31].
7. Inhaled Levosimendan
Levosimendan, when administered intravenously, can induce hypotension, which can be detrimental if not carefully managed, particularly in certain clinical settings. In response to this challenge, researchers have attempted to identify alternative administration routes, such as inhalation. This method presents a focused approach for achieving pulmonary vasodilation and may be effective for conditions such as pulmonary hypertension by reducing the risk of hypotension and decreasing the need for pressors [32].
The utility of inhaled levosimendan in the treatment of pulmonary hypertension has not been sufficiently understood because the available evidence is primarily based on a single pilot randomized double-blind studies. Kundra et al. investigated the impact of inhaled milrinone and levosimendan on pulmonary and systemic hemodynamics in 150 patients with coexisting pulmonary hypertension and mitral valve disease undergoing mitral valve surgery. The patients were divided into three groups: inhaled milrinone, inhaled levosimendan, and normal saline (control). They found that pulmonary artery pressure was significantly reduced in both milrinone and levosimendan groups compared to the placebo. Moreover, levosimendan exerted prolonged pulmonary vasodilatory effects (3 hours vs. milrinone’s 30 minutes), and both agents selectively dilated pulmonary vasculature without adverse effects [33].
Inhaled levosimendan holds great promise as a targeted pulmonary vasodilator, but its clinical translation faces several critical challenges. These include the difficulties in ensuring efficient pulmonary delivery, establishing optimal dosing regimens, and mitigating potential side effects. Investigations into bioavailability within the pulmonary vasculature and systemic circulation, coupled with thorough safety evaluations in various patient populations, are crucial to determining the viability of inhaled levosimendan in numerous treating conditions that require targeted pulmonary vasodilation. Although studies investigating the significance of inhaled levosimendan are still in early phases, preliminary data suggests that it can be applied in targeted pulmonary vasodilation, particularly in the context of pulmonary hypertension [34]. Thus, large-scale, randomized clinical trials are needed to determine the safety, efficacy, and optimal dosing protocol of inhaled levosimendan in various pulmonary conditions. Such studies can estabish its role as a viable treatment option for pulmonary hypertension.
8. Conclusion
The significance of levosimendan in the management of cardiovascular pharmacology continues to expand. This is evidenced by its application in diverse clinical settings, ranging from septic shock management to its role in perioperative cardiac surgery and intermittent outpatient use for patients with end-stage heart failure. The emergence of inhaled levosimendan has further broadened its therapeutic scope, presenting additional possibilities for cardiovascular care. On-going research and clinical trials are expected to offer valuable insights and strategies for optimizing levosimendan’s use in various cardiovascular conditions.
However, the present recommendations are primarily based on outcomes of randomized controlled trials. Therefore, further evidence is needed to support the use of levosimendan in various applications such as septic shock, cardiac surgery, end-stage heart failure, and the use of inhaled administration. This review highlights the drug’s extensive use in the treatment of cardiovascular diseases. Further research will increase our understanding of levosimendan’s mechanisms of action in specific clinical contexts thus can lay the foundation for personalized and effective treatment strategies. Given its increasing popularity as a second-line therapy for ADHF and a first-line option for cardiogenic shock, levosimendan is expected to become a hotspot in clinical research.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Farmakis, D.; Alvarez, J.; Gal, T.B.; et al. Levosimendan beyond inotropy and acute heart failure: Evidence of pleiotropic effects on the heart and other organs: An expert panel position paper. Int. J. Cardiol. 2016, 222, 303‒312. DOI: https://doi.org/10.1016/j.ijcard.2016.07.202
- Papp, Z.; Agostoni, P.; Alvarez, J.; et al. Levosimendan Efficacy and Safety: 20 years of SIMDAX in Clinical Use. J. Cardiovasc. Pharmacol. 2020, 76, 4‒22. DOI: https://doi.org/10.1097/FJC.0000000000000859
- Cholley, B.; Levy, B.; Fellahi, J.L.; et al. Levosimendan in the light of the results of the recent randomized controlled trials: an expert opinion paper. Crit Care. 2019, 23, 1‒8. DOI: https://doi.org/10.1186/s13054-019-2674-4
- Antila, S.; Pesonen, U.; Lehtonen, L.; et al. Pharmacokinetics of levosimendan and its active metabolite OR-1896 in rapid and slow acetylators. Eur. J. Pharm. Sci. 2004, 23, 213‒222. DOI: https://doi.org/10.1016/j.ejps.2004.07.005
- Conti, N.; Gatti, M.; Raschi, E.; et al. Evidence and Current Use of Levosimendan in the Treatment of Heart Failure: Filling the Gap. Drug Des. Devel Ther. 2021, 15, 3391‒3409. DOI: https://doi.org/10.2147/DDDT.S295214
- Pan, J.; Yang, Y.M.; Zhu, J.Y.; et al. Multiorgan Drug Action of Levosimendan in Critical Illnesses. Biomed Res. Int. 2019. 2019, 9731467. DOI: https://doi.org/10.1155/2019/9731467
- De Backer, D.; Cecconi, M.; Chew, M.S.; et al. A plea for personalization of the hemodynamic management of septic shock. Crit Care. 2022, 26, 372. DOI: https://doi.org/10.1186/s13054-022-04255-y
- Guan, Q.; Zhang, C.; Li, B.; et al. Meta-Analysis of the Efficacy of Levosimendan in the Treatment of Severe Sepsis Complicated with Septic Cardiomyopathy. Heart Surg. Forum 2023, 26, E609‒E620. DOI: https://doi.org/10.59958/hsf.6439
- Ravikumar, N.; Sayed, M.A.; Poonsuph, C.J.; et al. Septic Cardiomyopathy: From Basics to Management Choices. Curr. Probl. Cardiol. 2021, 46, 100767. DOI: https://doi.org/10.1016/j.cpcardiol.2020.100767
- Lima, M.R.; Silva, D. Septic cardiomyopathy: A narrative review. Revista Portuguesa de Cardiologia. 2023, 42, 471‒481. DOI: https://doi.org/10.1016/j.repc.2021.05.020
- Gordon, A.C.; Perkins, G.D.; Singer, M.; et al. Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N. Engl. J. Med. 2016, 375, 1638‒1648. DOI: https://doi.org/10.1056/NEJMoa1609409
- Meng, J.B.; Hu, M.H.; Lai, Z.Z.; et al. Levosimendan Versus Dobutamine in Myocardial Injury Patients with Septic Shock: A Randomized Controlled Trial. Med. Sci. Monit. 2016, 22, 1486‒1496. DOI: https://doi.org/10.12659/MSM.898457
- Ge, Z.; Gao, Y.; Lu, X.; et al. The association between levosimendan and mortality in patients with sepsis or septic shock: a systematic review and meta-analysis. Eur J Emerg Med. 2024, 31, 90‒97. DOI: https://doi.org/10.1097/MEJ.0000000000001105
- Di Molfetta, P.; Gregorini, R.; Fiore, C.; et al. Is There Still Room for the Prophylactic Use of Levosimendan in Cardiac Surgery? Ann. Thorac. Surg. 2018, 106, 1590. DOI: https://doi.org/10.1016/j.athoracsur.2018.05.007
- Landoni, G.; Lomivorotov, V.; Silvetti, S.; et al. Nonsurgical Strategies to Reduce Mortality in Patients Undergoing Cardiac Surgery: An Updated Consensus Process. J. Cardiothorac. Vasc. Anesth. 2018, 32, 225‒235. DOI: https://doi.org/10.1053/j.jvca.2017.06.017
- Landoni, G.; Lomivorotov, V.V.; Alvaro, G.; et al. Levosimendan for Hemodynamic Support after Cardiac Surgery. N. Engl. J. Med. 2017, 376, 2021‒2031. DOI: https://doi.org/10.1056/NEJMoa1616325
- Mehta, R.H.; Leimberger, J.D., van Diepen, S.; et al. Levosimendan in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. N. Engl. J. Med. 2017, 376, 2032‒2042. DOI: https://doi.org/10.1056/NEJMoa1616218
- Faisal, S.A.; Apatov, D.A.; Ramakrishna, H.; et al. Levosimendan in Cardiac Surgery: Evaluating the Evidence. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1146‒58. DOI: https://doi.org/10.1053/j.jvca.2018.05.035
- Ayala, R.; Gewehr, D.M.; Godoi, A.; et al. Preoperative Levosimendan in Patients with Severe Left Ventricular Dysfunction Undergoing Isolated Coronary Artery Bypass Grafting: A Meta-Analysis of Randomized Controlled Trials. J. Cardiothorac. Vasc. Anesth. 2024, 38, 649‒659. DOI: https://doi.org/10.1053/j.jvca.2023.11.036
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505‒1535. DOI: https://doi.org/10.1002/ejhf.1236
- Ferreira Reis, J.; Valentim Goncalves, A.; Ilhao Moreira, R.; et al. Levosimendan in outpatients with advanced heart failure: Single-center experience of 200 intermittent perfusions. Rev Port Cardiol. 2023, 42, 335‒343. DOI: https://doi.org/10.1016/j.repc.2022.03.006
- Altenberger, J.; Parissis, J.T.; Costard-Jaeckle, A.; et al. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: A multicentre randomized trial. Eur. J. Heart Fail. 2014, 16, 898‒906. DOI: https://doi.org/10.1002/ejhf.118
- Tycinska, A.; Gierlotka, M.; Bartus, S.; et al. Repetitive use of LEvosimendan in Ambulatory Heart Failure patients (LEIA-HF) - The rationale and study design. Adv. Med. Sci. 2022, 67, 18‒22. DOI: https://doi.org/10.1016/j.advms.2021.10.001
- Oliva, F.; Perna, E.; Marini, M.; et al. Scheduled intermittent inotropes for Ambulatory Advanced Heart Failure. The RELEVANT-HF multicentre collaboration. Int. J. Cardiol. 2018, 272, 255‒259. DOI: https://doi.org/10.1016/j.ijcard.2018.08.048
- Garcia-Gonzalez, M.J.; Aldea Perona, A.; Lara Padron, A.; et al. Efficacy and safety of intermittent repeated levosimendan infusions in advanced heart failure patients: the LAICA study. ESC Heart Fail. 2021, 8, 4820‒4831. DOI: https://doi.org/10.1002/ehf2.13670
- Comin-Colet, J.; Manito, N.; Segovia-Cubero, J.; et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur. J. Heart Fail. 2018, 20, 1128‒1136. DOI: https://doi.org/10.1002/ejhf.1145
- Oliva, F.; Comin-Colet, J.; Fedele, F.; et al. Repetitive levosimendan treatment in the management of advanced heart failure. Eur. Heart J. Suppl. 2018, 20, I11‒I20. DOI: https://doi.org/10.1093/eurheartj/suy040
- Codina, P.; Dobarro, D.; de Juan-Baguda, J.; et al. Heart failure risk scores in advanced heart failure patients: insights from the LEVO-D registry. ESC Heart Fail. 2023, 10, 2875‒2881. DOI: https://doi.org/10.1002/ehf2.14400
- Masarone, D.; Valente, F.; Verrengia, M.; et al. Efficacy and safety of repeated infusion of levosimendan in outpatients with advanced heart failure: a real-world experience. J. Cardiovasc. Med. 2020, 21, 919‒921. DOI: https://doi.org/10.2459/JCM.0000000000000983
- Polzl, G.; Allipour Birgani, S.; Comin-Colet, J.; et al. Repetitive levosimendan infusions for patients with advanced chronic heart failure in the vulnerable post-discharge period. ESC Heart Fail. 2019, 6, 174‒181. DOI: https://doi.org/10.1002/ehf2.12366
- Silvetti, S.; Pollesello, P.; Belletti, A. Repeated Levosimendan infusions in the management of advanced heart failure: review of the evidence and meta-analysis of the effect on mortality. J. Cardiovasc. Pharmacol. 2024, 83, 144–157. DOI: https://doi.org/10.1097/FJC.0000000000001506
- Hansen, M.S.; Andersen, A.; Nielsen-Kudsk, J.E. Levosimendan in pulmonary hypertension and right heart failure. Pulm. Circ. 2018, 8, 2045894018790905. DOI: https://doi.org/10.1177/2045894018790905
- Kundra, T.S.; Nagaraja, P.S.; Bharathi, K.S.; et al. Inhaled levosimendan versus intravenous levosimendan in patients with pulmonary hypertension undergoing mitral valve replacement. Ann. Card. Anaesth. 2018, 21, 328‒332. DOI: https://doi.org/10.4103/aca.ACA_19_18
- Evans, M.A.; Suresh, S. Inhaled levosimendan: New opportunities with an old drug. J. Clin. Anesth. 2021, 73, 110337. DOI: https://doi.org/10.1016/j.jclinane.2021.110337






