Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
The Application of Artificial Intelligence in the Research and Development of Traditional Chinese Medicine
Zhipeng Ke 1,2, Minxuan Liu 1,2,3, Jing Liu 1,2, Zhenzhen Su 1,2, Lu Li 1,2, Mengyu Qian 1,2, Xinzhuang Zhang 1,2, Tuanjie Wang 1,2, Liang Cao 1,2, Zhenzhong Wang 1,2, and Wei Xiao 1,2, *
1 National Key Laboratory on Technologies for Chinese Medicine Pharmaceutical Process Control and Intelligent Manufacture, Lianyungang 222106, China
2 Jiangsu Kanion Pharmaceutical Co., Ltd, Lianyungang 222104, China
3 School of Pharmacy, Nanjing University of Chinese Medicine, Nanjing 210009, China
* Correspondence: xw_kanion@163.com
Received: 4 September 2023
Accepted: 4 November 2023
Published: 18 March 2024
Abstract: With the accumulation of data in the pharmaceutical industry and the development of artificial intelligence technology, various artificial intelligence methods have been successfully employed in the drug discovery process. The integration of artificial intelligence in Traditional Chinese medicine has also gained momentum, encompassing quality control of Chinese patent medicines, prescriptions optimization, discovery of effective substances, and prediction of side effects. However, artificial intelligence also faces challenges and limitations in Traditional Chinese medicine development, such as data scarcity and complexity, lack of interdisciplinary professionals, black-box models, etc. Therefore, more research and collaboration are needed to address these issues and explore the best ways to integrate artificial intelligence and Traditional Chinese medicine to improve human health.
Keywords:
Artificial intelligence Chinese patent medicines Traditional Chinese medicine prescription quality control1. Introduction
Chinese people have greatly benefited from using Traditional Chinese medicine (TCM) in treating various illnesses, making it an important wealth to the Chinese nation [1,2]. Both the active ingredients extracted from TCM and the prescriptions composed of TCM have achieved excellent clinical efficacy. For example, Artemisinin extracted from Artemisia annua is a sesquiterpene lactone drug with anti-malignant malaria activity, saving millions of lives worldwide [3]. TCM prescriptions represented by “three TCM drugs and three herbal formulas” were essential in treating COVID-19 [4,5]. The potential efficacy of TCM with novel pharmacological mechanisms, low toxicity, and limited side effects has attracted increasing attention [6]. Unlike single-target synthetic drugs, TCM prescriptions generate therapeutic effects through complex interactions between components, targets, and pathways. Current experimental methods are limited to comprehensively studying and elucidating the molecular mechanisms of TCM [7]. Researchers have been attempting to develop TCM by employing modern intelligent technology recently [8].
Artificial intelligence (AI) stands as a dynamic and swiftly progressing domain, exerting a substantial influence across various sectors such as healthcare, finance, education, and security. AI incorporates innovative methods, such as natural language processing, machine learning, computational vision, robotics, and AI ethics, and has revolutionized drug design and discovery. Thanks to AI’s optimization of processes such as Quantitative Structure-Activity Relationship (QSAR) analysis, virtual screening, de novo drug design, and Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADME/T) evaluation, drug discovery is now more efficient, cost-effective, and effective than ever [9-11]. AI has already produced successful drug development cases in various therapeutic areas, such as the A2A receptor antagonist EXS-21546 created in just eight months by Evotec and Excientia. Similarly, Insilico Medicine’s AI program produced a new inhibitor (ISM001-055) for antifibrotic targets in nine months [12]. Despite these remarkable achievements, AI has yet to be widely applied to TCM, an ancient medical practice used for thousands of years and holds a wealth of knowledge and experience [13,14]. The combination of AI and TCM could lead to the creation of novel medicines that could benefit global healthcare. With the potential to optimize TCM’s efficacy and safety, AI has the power to transform the landscape of TCM research and development.
In this comprehensive review, we highlighted the multifaceted applications of AI in the quality control of Chinese patent medicines and the optimization of Traditional Chinese medicine prescriptions (Figure 1). We delve into the challenges facing artificial intelligence, emphasizing the prospects and future directions of this emerging field. The integration of AI with TCM charts the course for the development of innovative and globally impactful medicines. As the synergy between AI and TCM unfolds, it promises to unlock new dimensions in healthcare, seamlessly blending ancient wisdom with cutting-edge technology for the benefit of humanity.
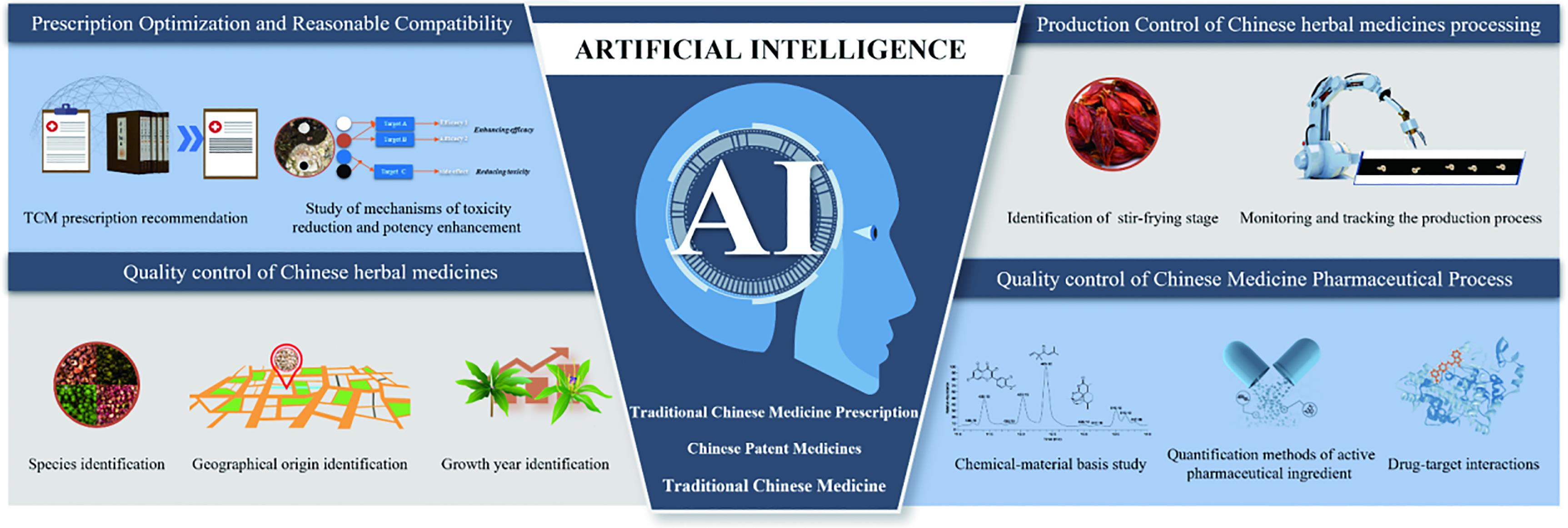
Figure 1. The application field of AI in the TCM research and development process..
2. Quality Control of Chinese Patent Medicines
TCM, especially Chinese Patent Medicines (CPMs), constitute a fundamental aspect of healthcare in China, playing a pivotal role in disease prevention and treatment [15]. The quality of CPMs is intrinsically linked to their effectiveness [16]. However, the production process of CPMs is often challenged by the heterogeneity of medicinal materials and pharmaceutical techniques, resulting in inconsistent product quality [17]. Variations in species, origin, harvest season, and processing methods of medicinal materials can lead to changes in their chemical composition and content, affecting the quality of Chinese medicine products [18-20]. The integration of AI technology offers potential solutions to these issues. Several studies have demonstrated the efficacy of AI in classifying different species of medicinal materials, predicting geographical origins, determining growth years, recognizing stir-frying stages, and enhancing recognition of types of Chinese Herbal Slices (CHS). AI has also been used to analyze active ingredients in CPMs, contributing to establishing quality control indicators. AI shows significant potential in addressing challenges and optimizing the quality control of CPMs.
In one instance, Tan et al. accomplished a remarkable 99.35% accuracy in the classification of various Zanthoxyli Pericarpium species, including Hanyuan ZB, Derong ZB, other Sichuan ZB, Zanthoxylum armatum, and Jinyang ZC, employing convolutional neural networks (CNNs) on image data [21]. In this study, the most advanced CNNs (including ResNet101, VGG16, DenseNets121 layers, and InceptionV4) and traditional machine learning algorithms (such as BP and SVM) were used to identify the types of Zanthoxyli Pericarpium. Notably, DenseNets121, due to its deep structure, exhibited susceptibility to overfitting during training, leading to reduced test accuracy. At the same time, VGG16’s simpler architecture limited its ability to extract high-level image semantics fully, causing lower accuracy on training and test datasets. Compared to traditional machine learning algorithms, as well as the CNNs models mentioned above, the proposed ResNet101 method outperforms them, exhibiting excellent performance, faster convergence, and enhanced recognition capabilities. This research’s success could pave the way for a novel approach to the rapid and precise classification of different species of medicinal materials. In another study, Wang et al. introduced hyperspectral imaging (HSI) assisted by an ACLSTM model, which incorporates an attention mechanism, CNNs, and long short-term memory, to predict the geographic origins of Coix seed (CS et al. var. ma-yuen (Roman.) Stapf) [22]. The proposed model in this study, which has the advantages of variable weight evaluation and continuous spectral information fusion, outperforms the conventional learning models and the individual deep learning module in predicting geographical origins. Selecting effective HSI wavelengths via the attention mechanism enhances prediction efficiency and results in interpretability. The method underscores the potential of HSI technology and ACLSTM for rapid, non-destructive quality evaluation of Coix seed. Yue et al. combined two-dimensional correlation spectroscopy (2DCOS) based on the attenuated total reflection Fourier transformed infrared spectroscopy (ATR-FTIR) with residual neural networks (ResNet) to accurately determine the growth years of Paris polyphylla var. Yunnanensis [23]. The study showcased the robust generalization ability of the synchronous 2DCOS model, which accurately identified different growth years with a 100% success rate. This makes it an ideal candidate for developing deep-learning models rooted in digital image processing. Furthermore, this research not only offers a reference point for determining the optimal harvesting period of Paris polyphylla var. yunnanensis but also introduces a practical method for evaluating similar medicinal plants. Zhang et al. proposed a method for recognizing the stir-frying stage of Gardeniae Fructus Praeparatus (GFP) using deep learning and transfer learning [24]. In this study, images of different stir-frying stages of GFP were trained using five pre-trained models: GoogLeNet, VGG16, ResNet34, MobileNetV2, and MobileNetV3. The results showed that all five models achieved an accuracy of over 95.82% on the test dataset. Among them, MobileNetV3 performed the best, having the fewest parameters and achieving the highest accuracy rate of 98.77%. This suggests that deep learning, particularly the lightweight network MobileNetV3, can effectively recognize the stir-frying stage of GFP. Wang et al. introduced a CCSM-Net, a novel network based on ResNeSt architecture, combining channel attention (CA) and spatial attention (SA) modules for enhanced recognition of local CHS images [25]. The CCSM-Net focuses on critical information in feature maps by leveraging both channel-wise and SA, with SA reinforcing CA’s capabilities in emphasizing spatial information. This approach enhances recognition accuracy without significantly increasing parameter sizes, making it a valuable tool for overseeing CHS production, enhancing automation, and improving traditional quality control methods in TCM production.
Under authentic Chinese herbal medicines and reasonable herb processing, active ingredients were analyzed using scientific methods. Quality control indicators based on these active ingredients were established to ensure that the quality of CPMs is consistent, stable, and controllable. For instance, a quality control strategy for Chinese medicine granules (CMGs), developed by Zhao, was established using machine learning and near-infrared spectroscopy (NIR) to analyze three active ingredients in sugar-free Yangwei granules rapidly [26]. NIR spectroscopy offers a non-destructive and efficient approach to CMG quality control. Given the complex relationship between NIR spectra and active ingredients in TCM, machine learning methods that handle nonlinear problems could be alternatives to Partial Least Squares (PLS) methods. Researchers applied three machine learning methods: the backpropagation artificial neural network (BP-ANN), interval PLS optimized by the genetic algorithm (GA-iPLS), and the particle swarm optimization-support vector machine (PSO-SVM) to construct predictive models. These models demonstrated exceptional performance and accuracy, highlighting that machine learning offers stability, predictability, and suitability for high-accuracy CMG analysis. In a study by Han et al., anti-inflammatory markers in various Flos Chrysanthemi (FC) were successfully identified [27]. They achieved this by developing a tandem method that combines Ultra Performance Liquid Chromatography-Quadrupole Time of Flight (UPLC-Q/TOF) with Principal Component Analysis (PCA) to differentiate the compositions in different FC types. Subsequently, a multilayer feed-forward Artificial Neural Network (ANN) with a single hidden layer was employed to illustrate the relationship between the optimal markers derived from PCA results and anti-inflammatory activity. This approach could be applied to the quality control of FC as well as other herbs and food items. Guo et al. crafted a meticulous quality assessment strategy for both the qualitative and quantitative analysis of constituents in TCM [28]. It included a novel deep-learning assisted mass defect filter (MDF) method for rapidly classifying mass spectrum ions from ultra-high performance liquid chromatography quadrupole time of flight tandem mass spectrometry (UHPLC-Q-TOF/MS). Additionally, a precise scheduled multiple reaction monitoring (sMRM) detection method was employed for accurate compound quantification. This integrated strategy offers an alternative avenue for exploring the chemical-material foundation of TCM and active ingredients.
Furthermore, numerous AI models and algorithms can predict drug-target interactions, including SSGCN [29], DeepChem [30], MT-DTI [31], NNScore [32], LigGrep [33], DeepDTA [31], and DeepAffinity [34]. These models are also effective in predicting natural products that work on molecular targets, which could potentially be the active ingredients of CPMs. Through these scientific methods, the active ingredients of CPMs could be identified, and then data mining and modeling technology were utilized to help optimize the extraction process of active ingredients. This helps to increase the content and quality of active ingredients in CPMs.
While AI has been extensively utilized in the quality control of CPMs, the scarcity of large-scale professional public datasets related to TCM for modeling and the accuracy of algorithms limits the ability of AI to learn a large amount of feature information [35]. This is partly due to the vast variety of medicinal materials, with approximately 13,000 types of herbs in China alone [36]. The Chinese Pharmacopoeia has established comprehensive standards for TCM, encompassing aspects such as origin, identification, testing and analysis, sample preparation, marker selection, and TCM processing [37]. These regulations ensure the quality control of TCM. However, the Pharmacopoeia only includes a limited number of medicinal materials, with the aim of ensuring the quality and safety of drugs. Many medicinal materials have not been standardized due to various factors including improvements to Pharmacopoeia standards, scarcity of resources, regional differences, clinical application values, and intellectual property issues. The author suggests referencing the quality assurance system for authentic medicinal materials in Sichuan to implement quality control throughout the entire process of herb cultivation and processing [38]. This would ensure the stable and uniform quality of all used medicinal materials and lay the foundation for the establishment of a standard database. At present, several databases for TCM are in use, including the Integrated Database of Traditional Chinese medicine (TCMID) 2.0, which was introduced in 2018 [39]. This database acts as an extensive repository, facilitating the modernization and standardization of TCM. The future research should focus on expanding the standard data volume for sufficient training and implementing appropriate data preprocessing to enhance the model’s generalization ability and accuracy [40,41].
In summary, AI holds substantial promise for enhancing the quality control, optimization, and effectiveness of CPMs, addressing challenges related to heterogeneity and quality assurance. Cross-disciplinary research and further data standardization efforts are essential for advancing these AI applications in CPMs.
3. Traditional Chinese Medicine Prescriptions Holistic Optimization and Synergy Strategies
The characteristic of TCM is to regulate the human body through multiple targets and pathways using components to treat the root cause of disease. This method aligns with modern medicine’s use of a combination of drugs to treat diseases effectively [2,40,42]. However, TCM prescriptions are not arbitrary combinations of drugs but are composed of several drugs based on summarized clinical experience and the principles of TCM compatibility to take advantage of each other and distinguish syndromes for treatment. It has been demonstrated in various instances that a combination of drugs can positively impact complicated diseases by enhancing their effectiveness and reducing toxicity [6,43]. Identifying and validating combinations can be challenging with the intricacy of TCM’s multi-component and multi-target. AI has emerged as a highly effective tool in the creation, evaluation, and refinement of TCM prescriptions [40]. AI not only provides recommendations for optimizing solutions by predicting drug toxicity, side effects, and synergistic effects but also contributes to a profound understanding of the intricate nature of TCM prescriptions.
For instance, Zhou et al. introduced FordNet, an innovative system that recommends TCM prescriptions [44]. This system processes electronic health record data using a deep neural network (DNN) algorithm. The research began by creating a CNN-based model, which was employed to extract intricate features from descriptions of disease diagnoses in clinical cases. Subsequently, based on the theory of TCM network pharmacology, an herb-compound-target heterogeneous network was constructed, and the network embedding (NE) method was applied to learn the deep features of TCM prescriptions, integrating active ingredients information. TCM network pharmacology dissects the molecular relationships between drugs and their therapeutic targets from a systemic and biological network perspective [42]. Combining, analyzing, and understanding macro and micro information would help better understand the rules of compatibility of TCM prescriptions. In the final phase of this research, a CNN-based intelligent recommendation method for TCM prescriptions was developed based on the extracted diagnostic description features and prescription characteristics. Compared to traditional methods like linear regression, FordNet exhibited a significant performance advantage. Notably, incorporating active ingredients information led to a remarkable 17.3% improvement in FordNet’s performance compared to models relying solely on macro-level data. This experiment is compelling evidence that the model effectively assimilates knowledge from experienced TCM practitioners and delivers commendable prescription recommendations.
In parallel, Dong et al. introduced a novel approach to TCM prescriptions recommendations, harnessing the power of subnetwork term mapping and deep learning [45]. This innovative fusion of techniques was strategically designed to confront the intricacies of TCM, ultimately elevating the precision and efficacy of TCM prescriptions. Ren et al. pioneered a prototype system for intelligently inheriting the profound wisdom of experienced TCM practitioners [46]. Impressively, this system achieved a prescription similarity exceeding 90% when compared to real-world prescriptions, highlighting AI’s potential not only to preserve but also to expand upon the expertise of seasoned TCM doctors. Furthermore, Zhao et al. ventured into the field by introducing a TCM prescriptions recommendation model grounded in a multi-graph convolutional network [47]. This method, which effectively utilizes the multi-graph convolutional network, is a versatile solution, significantly enhancing the precision of TCM prescriptions recommendations.
These collaborative pursuits of AI and traditional medical wisdom underscore the evolving landscape of medical practices and their potential to provide more effective healthcare solutions. However, AI-driven recommendations for TCM prescriptions face several key challenges. These encompass issues related to data quality, as inaccuracies or incomplete data can lead to erroneous recommendations and the limited availability of high-quality TCM datasets. Model interpretability is another hurdle, as opaque AI models hinder understanding and trust. Rigorous clinical validation is essential to verify the safety and efficacy of AI-recommended TCM prescriptions. Indeed, there is a growing body of research focused on addressing these challenges.
Some researchers have tried to reduce inaccuracies in AI-driven recommendations for TCM prescriptions through dataset improvement and model interpretability enhancements. For example, Liu et al. introduced TCMBERT, a two-stage transfer learning model designed to generate TCM prescriptions using a few medical records and TCM documentary resources [48]. Initially, ALBERT was employed to acquire TCM knowledge from sentence pairs extracted from TCM classics [48,49]. The encoder of ALBERT in this stage is extracted and combined with the Triple-Attention LSTM to construct the TCMBERT. This LSTM incorporated symptom and medical history inputs, enabling the model to learn from patient background information and symptom profiles. The outcome is a sequence of herbs in TCM prescriptions. These two stages are iteratively executed to enhance prescription quality. While the model performs strongly in learning from unstructured resources and understanding TCM principles and herb-symptom interactions, it may have limitations with small datasets, leading to reduced accuracy in recommending TCM prescriptions for certain symptoms. Nevertheless, the methods employed here offer insights for expanding AI learning samples.
To enhance the interpretability of herb recommendation, Jin et al. introduce a new model called Meta-path guided Graph Attention Network (MGAT) [50]. They emphasize the importance of understanding the underlying action mechanisms of herbs and develop an integrated Knowledge Graph that connects TCM theory with modern medicine’s pharmacology analysis. MGAT utilizes predefined meta-paths to guide information propagation and identifies long-range path instances with high attention weights to provide detailed explanations. Extensive experiments on a publicly available TCM dataset confirm MGAT’s effectiveness in uncovering herb action mechanisms while achieving comparable performance to state-of-the-art recommendation models and offering strong explainability.
Furthermore, ensuring reasonable compatibility is crucial for the safe and effective clinical use of TCM. When using TCM, there is a potential risk of adverse side effects resulting from interactions between herbs and drugs [51,52]. For example, the Chinese herbal medicine Rhubarb (Dahuang) has a two-way effect on liver protection and toxicity [52]. It can be combined with licorice, coptis, and other drugs to reduce toxicity. Studies have shown that glycyrrhizin, the main active ingredients in licorice, reduces rhubarb-induced liver damage through the Nrf2 pathway [53]. The study of mechanisms of toxicity reduction and potency enhancement through compatibility is a crucial element in interpreting the rationality of TCM compatibility.
To predict toxicity or side effects, Liu et al. used a previously developed framework and a deep learning method to predict toxicity or side effects to evaluate the safety of TCM prescriptions recommended for COVID-19 treatment in China [54]. Chen et al. developed a machine learning-based method for screening hepatotoxic compounds in TCM and Western medicine combinations, utilizing algorithms like support vector machines, neural networks, decision trees, and random forests [55]. In recent years, several successful AI models, including DeepHit, FP-ADMET, and ResNet18DNN, have been used to predict the toxicity properties of compounds like hERG, LD50, DILI, Ames mutagenesis, carcinogenesis, skin sensitization, and Tox21 assay endpoints [56]. Predicting the toxic components in Chinese medicine can help optimize the prescription, reduce toxicity, and enhance efficacy. In addition, drug-food constituent interactions (DFIs) could lead to adverse reactions or alter the intended efficiency of medications or therapies [57]. Therefore, using AI methods to predict the DFIs could provide some reference suggestions for the safe usage of TCM to ensure the efficacy of therapies. Quang-Hien Kha et al. utilized a variety of machine learning models and an MLP-based neural network classifier to perform training on datasets comprising drug-food pairs obtained from DrugBank. The results show that the XGBoost model had the highest performance, indicating the potential of this model to correctly detect different types of DFIs.
In the realm of AI-powered toxicity prediction, the intricate task of accommodating a multitude of toxicity attributes within a single model loom as a prominent challenge. The diverse nature of these attributes underscores the need for a multifaceted approach to enhance model performance. One promising avenue involves ensemble models, which amalgamate outputs from specialized models focusing on distinct toxicity attributes. This strategy yields a more comprehensive and all-encompassing perspective on toxicity prediction. Transfer learning represents another invaluable approach, allowing models to adapt pre-existing knowledge to toxicity prediction. This knowledge transfer significantly augments prediction accuracy. Additionally, regularly updating models with the latest research findings and data is essential to maintain their prominence in AI-driven toxicity prediction. In conclusion, addressing the comprehensive coverage of various toxicity attributes in AI-driven toxicity prediction calls for a versatile and dynamic approach. These strategies contribute to the enhancement and refinement of toxicity prediction models, ensuring their reliability and accuracy within this intricate domain.
Moreover, the combined use of multiple drugs with similar functions can amplify their synergistic effect and effectively lower the risk of toxic side effects. This is because using multiple similar drugs can reduce the dosage of each drug while achieving the same efficacy and even counteract some adverse effects of specific drugs [58]. One example of a highly toxic plant is Aconitum carmichaelii, which contains toxic diester diterpenoid alkaloids like aconitine and mesaconitine. However, by combining it with Zingiber officinale Roscoe and Glycyrrhiza uralensis Fisch ex DC, not only can it produce a significant curative effect, but it can also reduce its toxicity [43]. Aristolochic acid (AA) is known to cause health problems like aristolochic acid nephropathy and liver cancer. Nevertheless, when combined with berberine (Ber)-containing herbs, it becomes highly safe, suggesting that Ber neutralizes AA’s toxicity [59]. Two main methods are used to study synergistic relationships: the network-based approach and the machine-learning model prediction method [60,61]. Wang et al. used a network pharmacology framework to explore herb combinations more effectively and identify synergistic compound interactions based on network topology [62]. SynergyFinder 3.0 provides a novel synergy scoring method called Bliss/Loewe consensus that has become a widely used web tool for multi-drug combinatorial discovery and clinical translation [63]. Preuer et al. proposed a deep learning approach called DeepSynergy that integrated chemical descriptors and genomic data for predicting synergistic drug combinations [64]. Other deep learning models like SynPredict [65], DrugCombDB [66], and AuDNNsynergy [67] have also been suggested for prioritizing drug combinations with multi-omics data. However, these models mainly focus on predicting drug pairs and might not efficiently predict multi-drug synergies.
The field of predicting the synergistic effects of multiple drugs is highly complex, with existing models primarily focusing on predicting individual drug pairs, making them less efficient in predicting the synergy of various medicines. To address this challenge, researchers have put forward several strategies. Firstly, it is imperative to delve deeper into multi-drug interactions. This involves examining the combined effects of multiple drugs simultaneously, necessitating more extensive and more intricate datasets and more complex models. Secondly, the adoption of network-based approaches holds significant potential. Such methodologies offer the potential to furnish a deeper comprehension of the relationships between drugs, ultimately illuminating the possible mechanisms underlying multi-drug synergy. Moreover, the integration of data from multiple sources and diverse modalities, such as chemical structures, biological data, and text mining, is of paramount importance. This holistic approach permits a more thorough evaluation of the effects of drug combinations. Collectively, these strategies are poised to enhance model performance, enabling more accurate and reliable predictions of the synergy among multiple drugs.
In summary, the application of AI in TCM prescriptions recommendation and optimization holds the potential to find more effective solutions for TCM clinical practices. However, prior to achieving this goal, it is essential to address challenges such as data scarcity in models and the interpretability of the models.
4. Challenges and Outlook
The fusion of AI with TCM signifies a remarkable journey marked by challenges and promising prospects. This integration has ushered in advancements across diverse facets, from quality control of CPMs to TCM prescriptions optimization. However, this synergistic alliance encounters multifaceted challenges requiring a thoughtful and collaborative approach.
4.1. Data Challenges: Scarcity and Complexity
Integrating AI into TCM encounters a significant challenge due to the scarcity and complexity of data. Despite the rich knowledge of TCM, the available data often needs more completeness and accuracy for modern AI applications. TCM’s unique characteristics, involving numerous components, multiple targets, and a holistic approach, present a challenge for AI models that rely on comprehensive datasets. To fully harness the capabilities of AI in TCM research and development, addressing data scarcity and improving data quality is paramount. Notably, researchers have explored initiatives, such as implementing authentic medicinal material quality control throughout herb cultivation and processing, as referenced in the quality assurance system for authentic medicinal materials in Sichuan. This has resulted in the production of more standardized medicinal materials and the establishment of a standardized database. Additionally, efforts have been made to broaden information sources by incorporating electronic health record data, medical records, and TCM documentary resources.
4.2. Interdisciplinary Collaboration: Bridging Expertise Gaps
The successful integration of AI and TCM necessitates harmonious collaboration among professionals from diverse fields. The current absence of interdisciplinary experts proficient in both AI methodologies and the intricacies of TCM poses a challenge to seamless integration. Establishing a robust collaboration involving TCM practitioners, analytical chemists, biologists, statisticians, and AI experts is crucial. For instance, advanced separation and characterization techniques, along with AI technologies, are employed for both qualitative and quantitative research as well as quality control in TCM. When analytical chemists collaborate with AI experts, providing clear explanations of the data implications of separation and characterization technologies such as UPLC-Q/TOF, UHPLC-Q-TOF/MS is beneficial. This helps AI experts comprehend the experimental data obtained by analytical chemists, facilitating improved data processing, model establishment, and result interpretation. This collaborative synergy not only enhances the development process but also fosters a holistic understanding of the complex dynamics between AI and TCM.
4.3. Black-box Models: Balancing Complexity with Interpretability
The growing complexity of deep learning networks has led to the emergence of sophisticated yet opaque “black-box” models [68,69]. Despite their high performance, these models need more interpretability, impeding the understanding of their decision-making processes. The development of explainable AI models tailored to the TCM domain is essential for ensuring reliability and promoting a deeper comprehension of the insights provided by these models. Some researchers have integrated methods such as knowledge graphs and attention mechanisms to enhance the interpretability of models. Knowledge graphs represent and organize domain-specific knowledge, enabling a more structured understanding of the model. Attention mechanisms will allow the model to concentrate on crucial information in the input, leading to a more distinct elucidation of the model’s decision-making foundation. By combining these methods, AI models become more accessible for understanding and interpretation, enhancing their credibility in TCM.
4.4. Skepticism and Clinical Application: Building Trust Through Results
As AI technology evolves, its transformative impact on the TCM industry becomes increasingly apparent. Significant progress has been made in TCM and related fields, particularly in the development of intelligent diagnostic systems and personalized treatment plans through the application of AI. Examples include the use of AI to streamline the diagnosis of rare diseases, enhance the accuracy of cancer diagnoses, and provide personalized treatment approaches [70-73]. These advancements present advanced tools and methods for clinical practices in TCM. Nevertheless, challenges such as gaps in professional knowledge, data issues, and the opaqueness of black-box models contribute to persistent skepticism within the TCM industry regarding the applicability of AI. Overcoming this skepticism requires not only rigorous validation and clinical verification but also effective communication and collaboration between AI researchers and TCM practitioners. Establishing trust through tangible results and clear communication is crucial for the widespread acceptance of AI in the TCM landscape.
In conclusion, integrating AI with TCM holds immense promise, but its realization requires collaborative, interdisciplinary efforts. Overcoming data limitations, fostering transparency in AI models, and addressing industry skepticism are pivotal steps toward realizing the full potential of this synergistic partnership. As AI continues to unfold its capabilities, the outlook for the integration with TCM remains both challenging and promising, offering a transformative trajectory for healthcare research and development.
List of Abbreviation: TCM, Traditional Chinese medicine; AI, Artificial intelligence; QSAR, Quantitative Structure-Activity Relationship; CPMs, Chinese Patent Medicines; CHS, Chinese Herbal Slices; CNNs , convolutional neural networks; BP, backpropagation; SVM, support vector machine; ADME/T, Absorption, Distribution, Metabolism, Excretion, and Toxicity; HSI , hyperspectral imaging; 2DCOS, two-dimensional correlation spectroscopy; ATR-FTIR, attenuated total reflection Fourier transformed infrared spectroscopy; ResNet, residual neural networks; GFP, Gardeniae Fructus Praeparatus; CA, channel attention; SA, spatial attention; CMGs, Chinese medicine granules; NIR, near-infrared spectroscopy; PLS , Partial Least Squares; BP-ANN, backpropagation artificial neural network; GA-iPLS , interval PLS optimized by the genetic algorithm; PSO-SVM, particle swarm optimization-support vector machine; FC, Flos Chrysanthemi; UPLC-Q/TOF, Ultra Performance Liquid Chromatography-Quadrupole Time of Flight; PCA, Principal Component Analysis; ANN, Artificial Neural Network; UHPLC-Q-TOF/MS, ultra-high performance liquid chromatography quadrupole time of flight tandem mass spectrometry; MDF, mass defect filter; sMRM, scheduled multiple reaction monitoring; TCMID, Integrated Database of Traditional Chinese medicine; DNN, neural network; NE, network embedding; MGAT, Meta-path guided Graph Attention Network; DFIs, drug-food constituent interactions; AA, Aristolochic acid; Ber, berberine.
Author Contributions: Conceptualization, Zhipeng Ke, Xinzhuang Zhang and Liang Cao; resources, Jing Liu, Zhenzhen Su, Lu Li and Mengyu Qian; writing—original draft preparation, Zhipeng Ke; writing—review and editing, Minxuan Liu and Xinzhuang Zhang; project administration, Tuanjie Wang, Liang Cao, Zhenzhong Wang and Wei Xiao; All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by Programs Foundation for Leading Talents in National Administration of Traditional Chinese Medicine of China “Qihuang scholars” Project.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Wang, S.; Hou, Y.; Li, X.; et al. Practical implementation of artificial intelligence-based deep learning and cloud computing on the application of traditional medicine and western medicine in the diagnosis and treatment of rheumatoid arthritis. Front. Pharm. 2021, 12, 765435. DOI: https://doi.org/10.3389/fphar.2021.765435
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; et al. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1‒11. DOI: https://doi.org/10.1016/S1875-5364(21)60001-8
- Zhai, X.; Wang, Q.; Li, M. Tu Youyou’s Nobel Prize and the academic evaluation system in China. Lancet 2016, 387, 1722. DOI: https://doi.org/10.1016/S0140-6736(16)30261-6
- An, X.; Zhang, Y.; Duan, L.; et al. The direct evidence and mechanism of Traditional Chinese medicine treatment of COVID-19. Biomed. Pharmacother. 2021, 137, 111267. DOI: https://doi.org/10.1016/j.biopha.2021.111267
- Wang, W.Y.; Xie, Y.; Zhou, H.; et al. Contribution of Traditional Chinese medicine to the treatment of COVID-19. Phytomedicine 2021, 85, 153279. DOI: https://doi.org/10.1016/j.phymed.2020.153279
- Wei, Z.; Chen, J.; Zuo, F.; et al. Traditional Chinese medicine has great potential as candidate drugs for lung cancer: A review. J. Ethnopharmacol. 2023, 300, 115748. DOI: https://doi.org/10.1016/j.jep.2022.115748
- Zhang, B.; Wang, X.; Li, S. An integrative platform of tcm network pharmacology and its application on a herbal formula, Qing-Luo-Yin. Evid. Based Complement. Alternat. Med. 2013, 2013, 456747. DOI: https://doi.org/10.1155/2013/456747
- Li, N.; Yu, J.; Mao, X.; et al. The research and development thinking on the status of artificial intelligence in Traditional Chinese medicine. Evid. Based Complement. Alternat. Med. 2022, 2022, 7644524. DOI: https://doi.org/10.1155/2022/7644524
- Yang, X.; Wang, Y.; Byrne, R.; et al. Concepts of artificial intelligence for computer-assisted drug discovery. Chem. Rev., 2019, 119, 10520‒10594. DOI: https://doi.org/10.1021/acs.chemrev.8b00728
- Zhong, F.; Xing, J.; Li, X.; et al. Artificial intelligence in drug design. Sci. China Life Sci., 2018, 61, 1191‒1204. DOI: https://doi.org/10.1007/s11427-018-9342-2
- Tripathi, M.K.; Nath, A.; Singh, T.P.; et al. Evolving scenario of big data and Artificial Intelligence (AI) in drug discovery. Mol. Divers. 2021, 25, 1439‒1460. DOI: https://doi.org/10.1007/s11030-021-10256-w
- Jang, S.H.; Sivakumar, D.; Mudedla, S.K.; et al. PCW-A1001, AI-assisted de novo design approach to design a selective inhibitor for FLT-3(D835Y) in acute myeloid leukemia. Front. Mol. Biosci. 2022, 9, 1072028. DOI: https://doi.org/10.3389/fmolb.2022.1072028
- Zou, Y.; Shi, Y.; Sun, F.; et.al. Extreme gradient boosting model to assess risk of central cervical lymph node metastasis in patients with papillary thyroid carcinoma: Individual prediction using SHapley Additive exPlanations. Comput. Methods Programs Biomed. 2022, 225, 107038. DOI: https://doi.org/10.1016/j.cmpb.2022.107038
- Zhang, H.; Ni, W.; Li, J.; et al. Artificial intelligence-based traditional Chinese medicine Assistive Diagnostic System: Validation Study. JMIR Med. Inform. 2020, 8, e17608. DOI: https://doi.org/10.2196/17608
- Zhang, B.; Pei, W.; Cai, P.; et al. Recent advances in Chinese patent medicines entering the international market. Drug Discoveries Ther. 2022, 16, 258‒272. DOI: https://doi.org/10.5582/ddt.2022.01115
- Chen, H.; He, Y. Machine learning approaches in Traditional Chinese medicine: A systematic review. Am. J. Chin. Med. 2022, 50, 91‒131. DOI: https://doi.org/10.1142/S0192415X22500045
- Xue, Q.L.; Miao, K.H.; Yu, Y.; et al. Methodology for adaptive decision--making research on manufacturing process of Traditional Chinese medicine based on deep reinforcement learning. Zhong guo Zhong Yao Za Zhi, 2023, 48, 562‒568.
- He, X.; Huang, S.; Wu, M.; et al. Simultaneous quantitative analysis of ten bioactive flavonoids in Citri Reticulatae Pericarpium Viride (Qing Pi) by ultrahigh-performance liquid chromatography and high-resolution mass spectrometry combined with chemometric methods. Phytochem. Anal. 2021, 32, 1152‒1161. DOI: https://doi.org/10.1002/pca.3056
- Bai, C.; Yang, J.; Cao, B.; et al. Growth years and post-harvest processing methods have critical roles on the contents of medicinal active ingredients of Scutellaria baicalensis. Ind. Crops. Prod. 2020, 158, 112985. DOI: https://doi.org/10.1016/j.indcrop.2020.112985
- Zeng, P.; Li, J.; Chen, Y.; et al. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci., 2019, 163, 423‒444. DOI: https://doi.org/10.1016/bs.pmbts.2019.03.003
- Tan, C.; Wu, C.; Huang, Y.; et al. Identification of different species of Zanthoxyli Pericarpium based on convolution neural network. PLoS One 2020, 15, e0230287. DOI: https://doi.org/10.1371/journal.pone.0230287
- Wang, Y.; Xiong, F.; Zhang, Y.; et al. Application of hyperspectral imaging assisted with integrated deep learning approaches in identifying geographical origins and predicting nutrient contents of Coix seeds. Food Chem. 2023, 404, 134503. DOI: https://doi.org/10.1016/j.foodchem.2022.134503
- Yue, J.; Li, Z.; Zuo, Z.; et al. Study on the identification and evaluation of growth years for Paris polyphylla var. yunnanensis using deep learning combined with 2DCOS. Spectrochim. Acta, Part A 2021, 261, 120033. DOI: https://doi.org/10.1016/j.saa.2021.120033
- Zhang, Y.; Wang, C.; Wang, Y.; et al. Determining the stir-frying degree of gardeniae fructus praeparatus based on deep learning and transfer learning. Sensors 2022, 22, 8091. DOI: https://doi.org/10.3390/s22218091
- Wang, J.; Mo, W.; Wu, Y.; et al. Combined channel attention and spatial attention module network for chinese herbal slices automated recognition. Front. Neurosci. 2022, 16, 920820. DOI: https://doi.org/10.3389/fnins.2022.920820
- Zhao, J.; Tian, G.; Qiu, Y.; et al. Rapid quantification of active pharmaceutical ingredient for sugar-free Yangwei granules in commercial production using FT-NIR spectroscopy based on machine learning techniques. Spectrochim. Acta, Part A 2021, 245, 118878. DOI: https://doi.org/10.1016/j.saa.2020.118878
- Han, Y.; Zhou, M.; Wang, L.; et al. Comparative evaluation of different cultivars of Flos Chrysanthemi by an anti-inflammatory-based NF-kappaB reporter gene assay coupled to UPLC-Q/TOF MS with PCA and ANN. J. Ethnopharmacol. 2015, 174, 387‒395. DOI: https://doi.org/10.1016/j.jep.2015.08.044
- Guo, J.; Zhang, L.; Shang, Y.; et al. A strategy for intelligent chemical profiling-guided precise quantitation of multi-components in Traditional Chinese medicine formulae-QiangHuoShengShi decoction. J. Chromatogr. A. 2021, 1649, 462178. DOI: https://doi.org/10.1016/j.chroma.2021.462178
- Zhong, F.; Wu, X.; Yang, R.; et al. Drug target inference by mining transcriptional data using a novel graph convolutional network framework. Protein Cell 2022, 13, 281‒301. DOI: https://doi.org/10.1007/s13238-021-00885-0
- Zhu, H. Big data and artificial intelligence modeling for drug discovery. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 573‒589. DOI: https://doi.org/10.1146/annurev-pharmtox-010919-023324
- Beck, B.R.; Shin, B.; Choi, Y.; et al. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020, 18, 784‒790. DOI: https://doi.org/10.1016/j.csbj.2020.03.025
- Serafim, M.S.M.; Kronenberger, T.; Oliveira, P.R.; et al. The application of machine learning techniques to innovative antibacterial discovery and development. Expert. Opin, Drug Discov. 2020, 15, 1165‒1180. DOI: https://doi.org/10.1080/17460441.2020.1776696
- Spiegel, J.O.; Durrant, J.D. AutoGrow4: an open-source genetic algorithm for de novo drug design and lead optimization. J. Cheminform. 2020, 12, 25. DOI: https://doi.org/10.1186/s13321-020-00429-4
- Karimi, M.; Wu, D.; Wang, Z.; et al. DeepAffinity: interpretable deep learning of compound-protein affinity through unified recurrent and convolutional neural networks. Bioinformatics 2019, 35, 3329‒3338. DOI: https://doi.org/10.1093/bioinformatics/btz111
- Lu, S.H.; Zhang, M.C.; Zhai, H.L.; et al. Rapid determination in the quality control of Chinese patent medicine. J. Pharm. Innov. 2022, 17, 1305‒1313. DOI: https://doi.org/10.1007/s12247-021-09608-8
- Keji, C.; Bei, Y. Certain progress of clinical research of Chinese integrative medicine. Chin. Med. J. 1999, 112, 934‒937.
- Leong, F.; Hua, X.; Wang, M.; et al. Quality standard of Traditional Chinese medicines: comparison between European Pharmacopoeia and Chinese Pharmacopoeia and recent advances. Chin. Med. 2020, 15, 76. DOI: https://doi.org/10.1186/s13020-020-00357-3
- Luo, H.; Zhao, Y.; Hua, H.; et al. Research progress on quality assurance of genuine Chinese medicinal in Sichuan. Chin. Med. 2021, 16, 19. DOI: https://doi.org/10.1186/s13020-021-00428-z
- Huang, L.; Xie, D.; Yu, Y.; et al. TCMID 2.0: A comprehensive resource for TCM. Nucleic. Acids. Res. 2018, 46, D1117‒D1120. DOI: https://doi.org/10.1093/nar/gkx1028
- Li, D.; Hu, J.; Zhang, L.; et al. Deep learning and machine intelligence: New computational modeling techniques for discovery of the combination rules and pharmacodynamic characteristics of Traditional Chinese medicine. Eur. J. Pharmacol. 2022, 933, 175260. DOI: https://doi.org/10.1016/j.ejphar.2022.175260
- Gupta, R.; Srivastava, D.; Sahu, M.; et al. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315‒1360. DOI: https://doi.org/10.1007/s11030-021-10217-3
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110‒120. DOI: https://doi.org/10.1016/S1875-5364(13)60037-0
- Chen, H.; Huang, Y.; Liu, H.; et al. Research progress on the mechanism of reducing toxicity and increasing the efficacy of sini decoction compatibility. Chem. Pharm. Bull. 2022, 70, 827‒838. DOI: https://doi.org/10.1248/cpb.c22-00380
- Zhou, W.; Yang, K.; Zeng, J.; et al. FordNet: Recommending traditional Chinese medicine formula via deep neural network integrating phenotype and molecule. Pharmacol. Res. 2021, 173, 105752. DOI: https://doi.org/10.1016/j.phrs.2021.105752
- Dong, X.; Zheng, Y.; Shu, Z.; et al. TCMPR: TCM prescription recommendation based on subnetwork term mapping and deep learning. Biomed. Res. Int. 2022, 2022, 4845726. DOI: https://doi.org/10.1155/2022/4845726
- Ren, X.; Guo, Y.; Wang, H.; et al. The intelligent experience inheritance system for Traditional Chinese medicine. J. Evid. Based. Med. 2023, 16, 91‒100. DOI: https://doi.org/10.1111/jebm.12517
- Zhao, W.; Lu, W.; Li, Z.; et. al. TCM herbal prescription recommendation model based on multi-graph convolutional network. J. Ethnopharmacol. 2022, 297, 115109. DOI: https://doi.org/10.1016/j.jep.2022.115109
- Liu, Z.; Luo, C.; Fu, D.; et al. A novel transfer learning model for traditional herbal medicine prescription generation from unstructured resources and knowledge. Artif. Intell. Med. 2022, 124, 102232. DOI: https://doi.org/10.1016/j.artmed.2021.102232
- Lan, Z.; Chen, M.; Goodman, S.; et al. ALBERT: A lite bert for self-supervised learning of language Representations. arXiv preprint arXiv 2019, 1909, 11942. https://doi.org/10.48550/arXiv.1909.11942.
- Jin, Y.; Ji, W.; Shi, Y.; et al. Meta-path guided graph attention network for explainable herb recommendation. Health. Inf. Sci. Syst. 2023, 11, 5. DOI: https://doi.org/10.1007/s13755-022-00207-6
- Parvez, M.K.; Rishi, V. Herb-Drug Interactions and Hepatotoxicity. Curr. Drug Metab. 2019, 20, 275‒282. DOI: https://doi.org/10.2174/1389200220666190325141422
- Zhuang, T.; Gu, X.; Zhou, N.; et al. Hepatoprotection and hepatotoxicity of Chinese herb Rhubarb (Dahuang): How to properly control the “General (Jiang Jun)” in Chinese medical herb. Biomed. Pharmacother. 2020, 127, 110224. DOI: https://doi.org/10.1016/j.biopha.2020.110224
- Ni, B.; Liu, Y.; Gao, X.; et al. Isoliquiritigenin attenuates emodin-induced hepatotoxicity in vivo and in vitro through Nrf2 pathway. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2022, 261, 109430. DOI: https://doi.org/10.1016/j.cbpc.2022.109430
- Wang, Z.; Li, L.; Song, M.; et al. Evaluating the Traditional Chinese medicine (TCM) Officially Recommended in China for COVID-19 Using Ontology-Based Side-Effect Prediction Framework (OSPF) and Deep Learning. J. Ethnopharmacol. 2021, 272, 113957. DOI: https://doi.org/10.1016/j.jep.2021.113957
- Chen, Z.; Zhao, M.; You, L.; et al. Developing an artificial intelligence method for screening hepatotoxic compounds in Traditional Chinese medicine and Western medicine combination. Chin. Med. 2022, 17, 58. DOI: https://doi.org/10.1186/s13020-022-00617-4
- Tran, T.T.V.; Surya, Wibowo A.; Tayara, H.; et al. Artificial Intelligence in Drug Toxicity Prediction: Recent Advances, Challenges, and Future Perspectives. J. Chem. Inf. Model. 2023, 63, 2628‒2643. DOI: https://doi.org/10.1021/acs.jcim.3c00200
- Kha, Q.H.; Le, V.H.; Hung, T.N.K.; et al. Development and Validation of an Explainable Machine Learning-Based Prediction Model for Drug-Food Interactions from Chemical Structures. Sensors 2023, 23, 3962. DOI: https://doi.org/10.3390/s23083962
- Xie, Y.; Mai, C.T.; Zheng, D.C.; et al. Wutou decoction ameliorates experimental rheumatoid arthritis via regulating NF-kB and Nrf2: Integrating efficacy-oriented compatibility of Traditional Chinese medicine. Phytomedicine 2021, 85, 153522. DOI: https://doi.org/10.1016/j.phymed.2021.153522
- Wang, P.; Guo, W.; Huang, G.; et al. Berberine-Based heterogeneous linear supramolecules neutralized the acute nephrotoxicity of aristolochic acid by the self-assembly strategy. ACS Appl. Mater. Interfaces. 2021, 13, 32729‒32742. DOI: https://doi.org/10.1021/acsami.1c06968
- Kuenzi, B.M.; Park, J.; Fong, S.H.; et al. Predicting drug response and synergy using a deep learning model of human cancer cells. Cancer Cell 2020, 38, 672‒684.e6. DOI: https://doi.org/10.1016/j.ccell.2020.09.014
- Cheng, F.; Kovacs, I.A.; Barabasi, A.L. Network-based prediction of drug combinations. Nat. Commun. 2019, 10, 1197. DOI: https://doi.org/10.1038/s41467-019-09186-x
- Wang, Y.; Yang, H.; Chen, L.; et al. Network-based modeling of herb combinations in Traditional Chinese medicine. Brief. Bioinform. 2021, 22, bbab106. DOI: https://doi.org/10.1093/bib/bbab106
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic. Acids. Res. 2022, 50, W739‒W743. DOI: https://doi.org/10.1093/nar/gkac382
- Preuer, K.; Lewis, R.P.I.; Hochreiter, S.; et al. DeepSynergy: predicting anti-cancer drug synergy with deep learning. Bioinformatics 2018, 34, 1538‒1546. DOI: https://doi.org/10.1093/bioinformatics/btx806
- Alsherbiny, M.A.; Radwan, I.; Moustafa, N.; et al. Trustworthy deep neural network for inferring anticancer synergistic combinations. IEEE J. Biomed. Health Inform. 2023, 27, 1691‒1700. DOI: https://doi.org/10.1109/JBHI.2021.3126339
- Liu, H.; Zhang, W.; Zou, B.; et al. DrugCombDB: A comprehensive database of drug combinations toward the discovery of combinatorial therapy. Nucleic. Acids. Res. 2020, 48, D871‒D881. DOI: https://doi.org/10.1093/nar/gkz1007
- Zhang, T.; Zhang, L.; Payne, P.R.O.; et al. Synergistic drug combination prediction by integrating multiomics data in deep learning models. Methods Mol Biol. 2021, 2194, 223‒238. DOI: https://doi.org/10.1007/978-1-0716-0849-4_12
- Nguyen, T.M.; Quinn, T.P.; Nguyen, T.; et al. Explaining black box drug target prediction through model agnostic counterfactual samples. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 1020‒1029. DOI: https://doi.org/10.1109/TCBB.2022.3190266
- Vo, T.H.; Nguyen, N.T.K.; Kha, Q.H.; et al. On the road to explainable AI in drug-drug interactions prediction: A systematic review. Comput. Struct. Biotechnol. J. 2022, 20, 2112‒2123. DOI: https://doi.org/10.1016/j.csbj.2022.04.021
- Nguyen, H.S.; Ho, D.K.N.; Nguyen, N.N.; et al. Predicting EGFR mutation status in non-small cell lung cancer using artificial intelligence: a systematic review and meta-analysis. Acad. Radiol. 2023. DOI: https://doi.org/10.1016/j.acra.2023.03.040
- Wojtara, M.; Rana, E.; Rahman, T.; et al. Artificial intelligence in rare disease diagnosis and treatment. Clin. Transl. Sci. 2023, 16, 2106‒2111 DOI: https://doi.org/10.1111/cts.13619
- Chen, Z.H.; Lin, L.; Wu, CF.; et al. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun. 2021, 41, 1100‒1115. DOI: https://doi.org/10.1002/cac2.12215
- Blasiak, A.; Khong, J.; Kee, T. CURATE.AI: Optimizing personalized medicine with artificial intelligence. SLAS. Techno. 2020, 25, 95‒105. DOI: https://doi.org/10.1177/2472630319890316






