Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
Effect of Glucagon-like Peptide-1 Receptor Agonist on Cardiac Structure and Function in Patients with Heart Failure: A Systematic Review and Meta-analysis
Xinyu Zhang 1, and Hongyuan Zhang 2, *
1 Division of Bioscience, University College London, London, UK
2 Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK
* Correspondence: hongyuan.zhang-3@postgrad.manchester.ac.uk
Received: 23 May 2023
Accepted: 8 August 2023
Published: 28 September 2023
Abstract: Recent clinical trials have shown that glucagon-like peptide-1 receptor agonists (GLP-1RAs) yield positive effects on composite cardiovascular endpoints, rendering them potentially promising therapeutic agents for heart failure (HF). This study analysed the effect of GLP-1RAs on cardiac structure and function in HF patients. MethodsA comprehensive search was conducted across PubMed, Cochrane Library, Ovid Embase, Ovid Medline, and Web of Science databases, spanning from inception to August 1, 2022, to identify randomised controlled trials (RCTs) comparing alterations in cardiac structure and function in HF patients receiving GLP-1RAs or placebo. Cardiac structures were assessed through left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), and left ventricular mass (LVM). Systolic function was evaluated using left ventricular ejection fraction (LVEF), stroke volume (SV), and global longitudinal strain (GLS). Diastolic function was assessed via the early to late diastolic filling velocity ratio (E/A ratio) and the early transmitral filling velocity to early diastolic mitral annular velocity ratio (E/e ratio). The I2 statistic was used to test heterogeneity. Pooled relative risks were calculated using random-effects models. Potential publication bias was assessed using the Cochrane Risk of Bias 2 tool. ResultsA total of 1,417 patients from 16 randomised placebo-controlled trials were enrolled in this meta-analysis. Among all HF patients, GLP-1RAs demonstrated improvement in diastolic function as indicated by E/A (MD = -0.15; 95% CI: -0.21 to -0.09; P < 0.00001; I2 = 43%) and E/e’ (MD = -0.82; 95% CI: -1.53 to -0.11; P = 0.02; I2= 62%). However, GLP-1RAs did not exhibit any improvement in cardiac structure and systolic function parameters for HF patients. ConclusionGLP-1RAs demonstrated potential for improving diastolic function in HF patients, but did not show any impact on systolic function and cardiac structure. Therefore, the application of GLP-1RAs should be based on the specific HF type and accompanying comorbidities.
Keywords:
Glucagon-like peptide-1 receptor heart failure cardiac function cardiac structure1. Introduction
Cardiovascular disease (CVD) stands as a prominent global cause of mortality [1]. As CVD survival rates increase and populations age, many cases progress into heart failure (HF) and ultimately result in cardiac-related deaths [2]. Type 2 diabetes mellitus (T2DM) constitutes a significant risk factor for HF, often exacerbating morbidity and mortality in HF patients [3]. The prevalence of T2DM among HF patients ranges from 40% to 50%, while HF prevalence among T2DM patients is estimated at 20% [4-7]. The updated guidelines from the American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) classify HF into three types based ejection fraction: HF with reduced ejection fraction (HFrEF), HF with preserved ejection fraction (HFpEF), and HF with middle range ejection fraction (HFmrEF). First-line pharmacological treatments for HFrEF include angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEIs), mineralocorticoid receptor antagonists (MRAs), and beta-blockers (BBs), which have demonstrated reductions in morbidity and mortality [8,9]. Recent guideline updates introduced two new classes of medications: the sinoatrial node modulator ivabradine [10] and the angiotensin receptor-neprilysin inhibitor valsartan/sacubitril (ARNI). Recent clinical trial outcomes indicate that sodium-glucose cotransporter 2 inhibitors (SGLT2i) [11-13], soluble guanylate cyclase stimulator vericiguat [14], and the cardiac-specific myosin activator omecamtiv-mecarbil [15] further improve HFrEF outcomes.
Glucose-like-peptide 1 receptor agonists (GLP-1RAs) are a class of anti-diabetic drugs that have recently shown cardiovascular benefits. Numerous guidelines now strongly recommend GLP-1RAs for T2DM patients with CVD [16,17]. A recent meta-analysis of eight large cardiovascular outcome trials (CVOTs) highlighted GLP-1RAs’ moderate reduction in major adverse cardiac events (MACE), alongside decreases in all-cause mortality and HF-related hospitalisation [18].
Although there have been several reviews and meta-analyses on the effect of GLP-1RAs on left ventricular function and structure [19-21], an updated meta-analysis is warranted due to emerging randomised controlled trials (RCTs). Therefore, the current review aims to analyse left ventricular function parameters among HF patients receiving GLP-1RA therapy, ultimately evaluating GLP-1RAs’ effects on HF.
2. Methods
2.1. Protocol
This meta-analysis adhered to the updated guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) [22]. The review was registered in the National Institute for Health Research international prospective register of systematic reviews (PROSPERO) (CRD42022374881).
2.2. Data Source and Search Strategy
The PICOS tool was utilized to define the scope of the literature. Randomized controlled studies involving HF patients (population) were included, and comparisons were made between patients administered GLP-1RA (intervention) and those receiving a placebo (comparison). This study assessed heart systolic and diastolic function outcomes.
A comprehensive search was conducted across five databases: PubMed, Cochrane Library, Ovid Embase, Ovid Medline, and Web of Science from inception to August 1, 2022. The search strategy was based on a search formula combining GLP-1RAs and HF-related terms. The following keywords were employed: (GLP-1 agonist OR glucagon-like peptide-1 receptor agonists OR lixisenatide OR liraglutide OR semaglutide OR exenatide OR albiglutide OR dulaglutide OR efpeglenatide) AND (cardiac failure OR heart decompensation OR decompensation, heart OR heart failure, right-sided OR heart failure, right sided OR right-sided heart failure OR right sided heart failure OR myocardial failure OR congestive heart failure OR heart failure, congestive OR heart failure, left-sided OR heart failure, left sided OR left-sided heart failure OR left sided heart failure OR heart failure). These keywords were applied to a full-text search in all languages and the most recent updates. The reference list of included articles and relevant meta-analyses were scrutinized to identify additional potentially relevant studies.
2.3. Study Selection and Eligibility Criteria
Two independent reviewers (Xinyu and Hongyuan) screened titles, abstracts, and full texts using Covidence. This meta-analysis included RCTs that compared GLP-1RAs with a control group and reported cardiac function outcomes, including systolic and diastolic function. Both anatomical aspects and functional metrics of systolic and diastolic function were assessed using echocardiography and cardiac magnetic resonance. Conference abstracts, non-RCTs, observational/retrospective studies, studies comparing GLP-1RAs with non-control groups (other drugs such as SGLT2 inhibitor), and studies that did not report cardiac function outcomes were excluded.
2.4. Data Extraction
Data from each eligible study were extracted into a standardised form, including study identifies (author, year of publication, and PubMed identification (PMID)), population details (sample size in experimental and placebo, age, gender, body mass index (BMI) and disease history), intervention (name of drugs, doses, and intervention time), and cardiac function outcomes (heart rate, anatomical parameters, systolic and diastolic function).
2.5. Risk of Bias Assessment
Two reviewers independently conducted a risk of bias assessment using the Cochrane Risk of Bias 2 (ROB2) tool, which assesses five domains: randomisation process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. Each domain was categorized as low risk, some concerns, or high risk.
2.6. Statistical Analysis
The extracted data were analysed using RevMan version 5.4. Pooled outcomes were derived using the inverse variance method, and the random-effects model was used to account for between-study variability. For studies lacking standard deviations (SDs), 95% confidence intervals (CIs), and interquartile ranges (IQRs) were converted to SDs according to the Cochrane Handbook for Systematic Reviews of Interventions. Statistical heterogeneity was assessed using Cochrane’s Q test via the chi‐square test and further quantified by the I 2 statistic. I 2 values of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively. Two-sided P-values <0.05 were considered statistically significant.
3. Results
3.1. Study Selection and Patient Characteristics
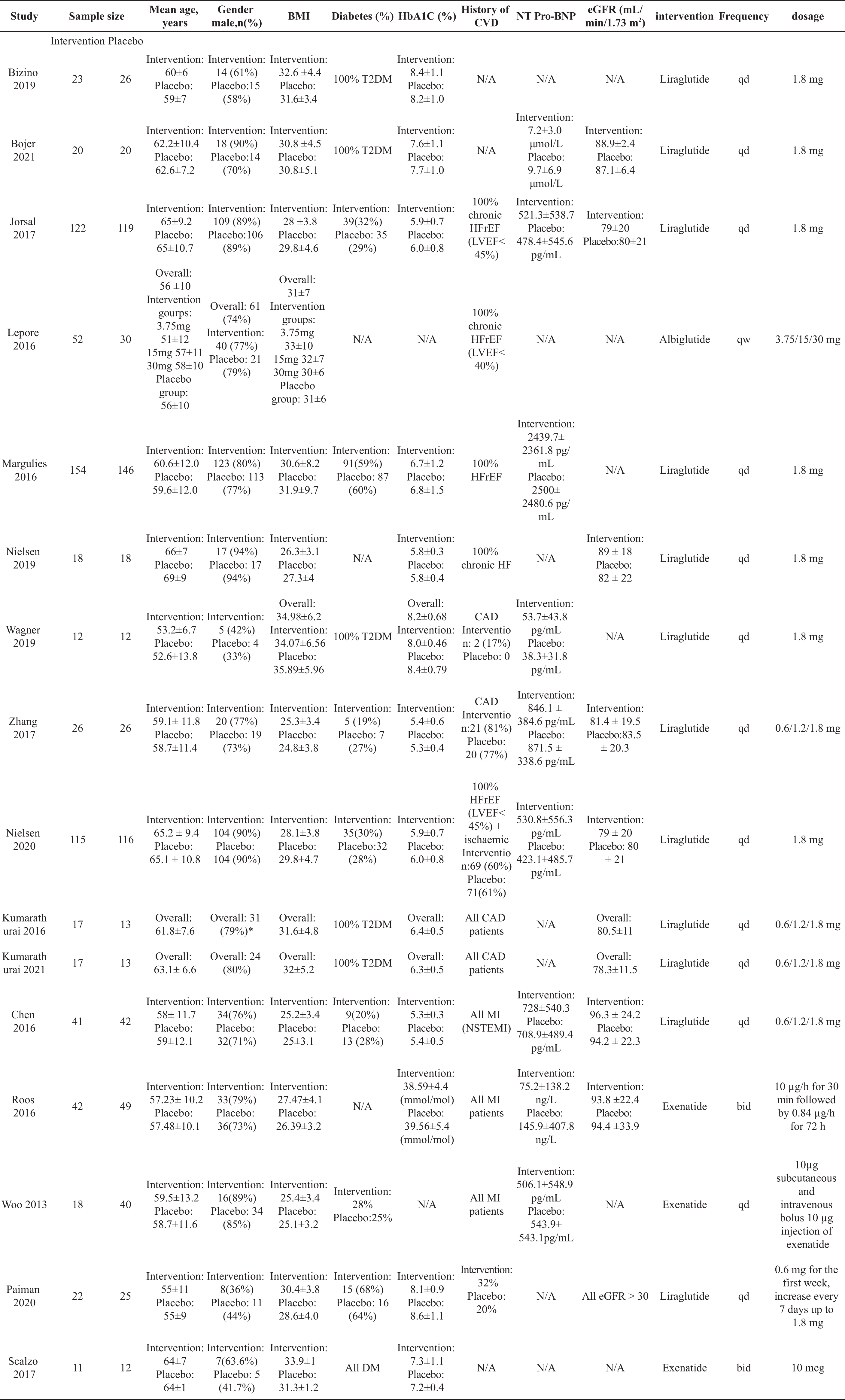
Figure 1 presents the flowchart detailing the study selection process. A total of 3,605 references were identified through electronic searches, out of which 1,543 duplicates were eliminated. Subsequently, 2,011 studies were excluded after a thorough review of their titles and abstracts. Upon careful examination of the full texts, 42 studies were further excluded. Ultimately, 16 studies, comprising 1,417 patients met the inclusion criteria and were included in both the qualitative synthesis and the meta-analysis [23-38]. All eligible studies adhered to the RCT design, and the risk of bias assessment by Rob2.0 revealed no studies with high risk (Figure 2). The baseline clinical characteristics of the patients included in this meta-analysis are summarised in Table 1. Among the included studies, liraglutide was examined in twelve studies [23-25, 27-34, 37], exenatide was investigated in three studies [35, 36, 38], and albiglutide was explored in one study [26]. Notably, the distribution of patients was well-balanced between the placebo and intervention groups except for one study [36], where 40 patients received a placebo while 18 patients were assigned to the intervention group. Among the enrolled patients, male participants predominated in the majority of studies [23-28, 30-36, 38], except for two studies where female patients were the majority [29, 37]. Across 15 studies [23-29, 31-38] the average body mass index (BMI) exceeded 25 kg/m2, thus aligning with the World Health Organization (WHO) definition of obesity. Assessment of cardiac function was conducted using echocardiography and cardiac magnetic resonance.
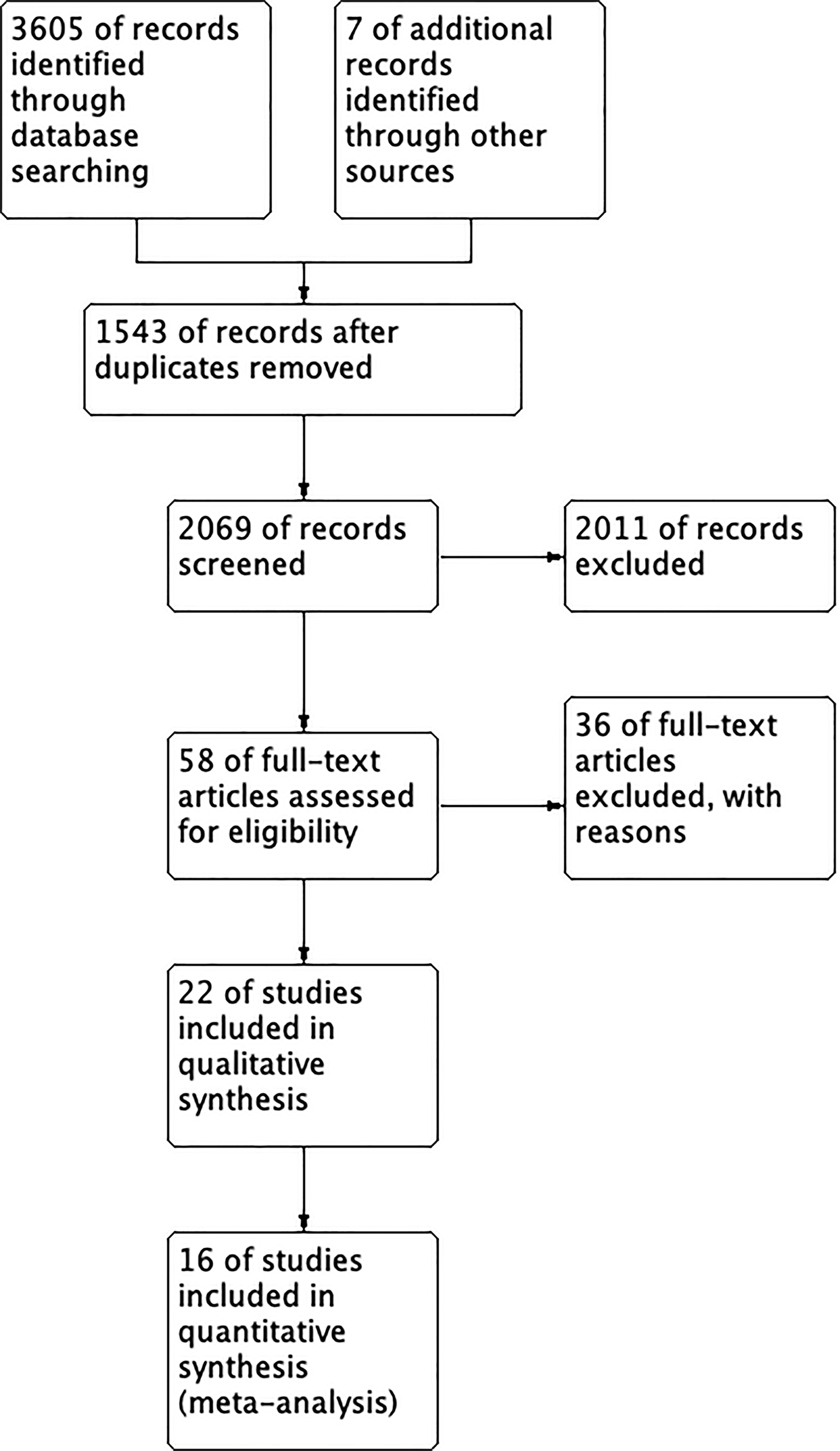
Figure 1.
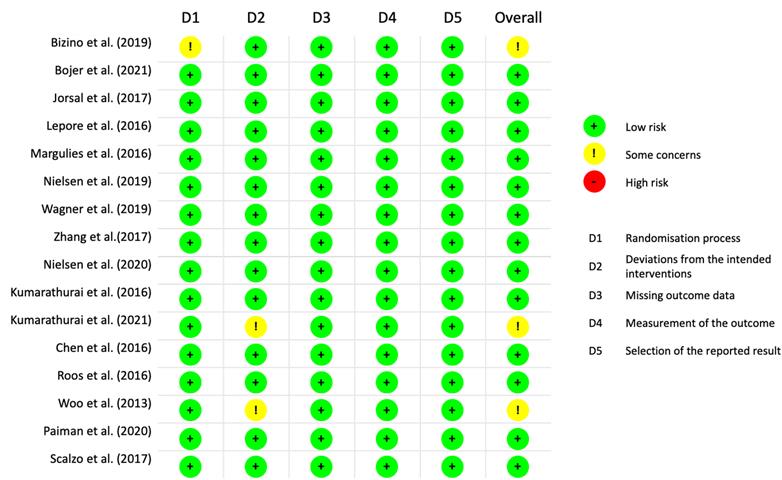
Figure 2.
Table 1. The characteristics of patients included in the meta‐analysis
3.2. Effect of GLP-1RAs on Cardiac Structure
Cardiac structural parameters were evaluated through the assessment of left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), and left ventricular mass (LVM). The comparison was drawn between HF patients receiving GLP-1RAs and those administered a placebo (Figure 3A-3C). A comprehensive analysis of ten studies [23-27, 30, 34-37], involving a total of 520 HF patients on GLP-1RAs and 523 HF patients on placebo, revealed no statistically significant differences between the two groups in terms of LVESV (mean difference (MD) = -0.01; 95% CI: -2.57 to 2.55; P = 0.99; I 2 = 74%) and LVEDV (MD = -3.11; 95% CI: -6.85 to 0.62; P = 0.10; I 2 = 78% ) (Figure 3A, 3B). Similarly, an assessment of six studies [23, 24, 29, 34, 35, 37] revealed no significan difference in LVM between the two groups (MD = -2.93; 95% CI: -7.76 to 1.89; P = 0.23; I 2 = 52% ) (Figure 3C).
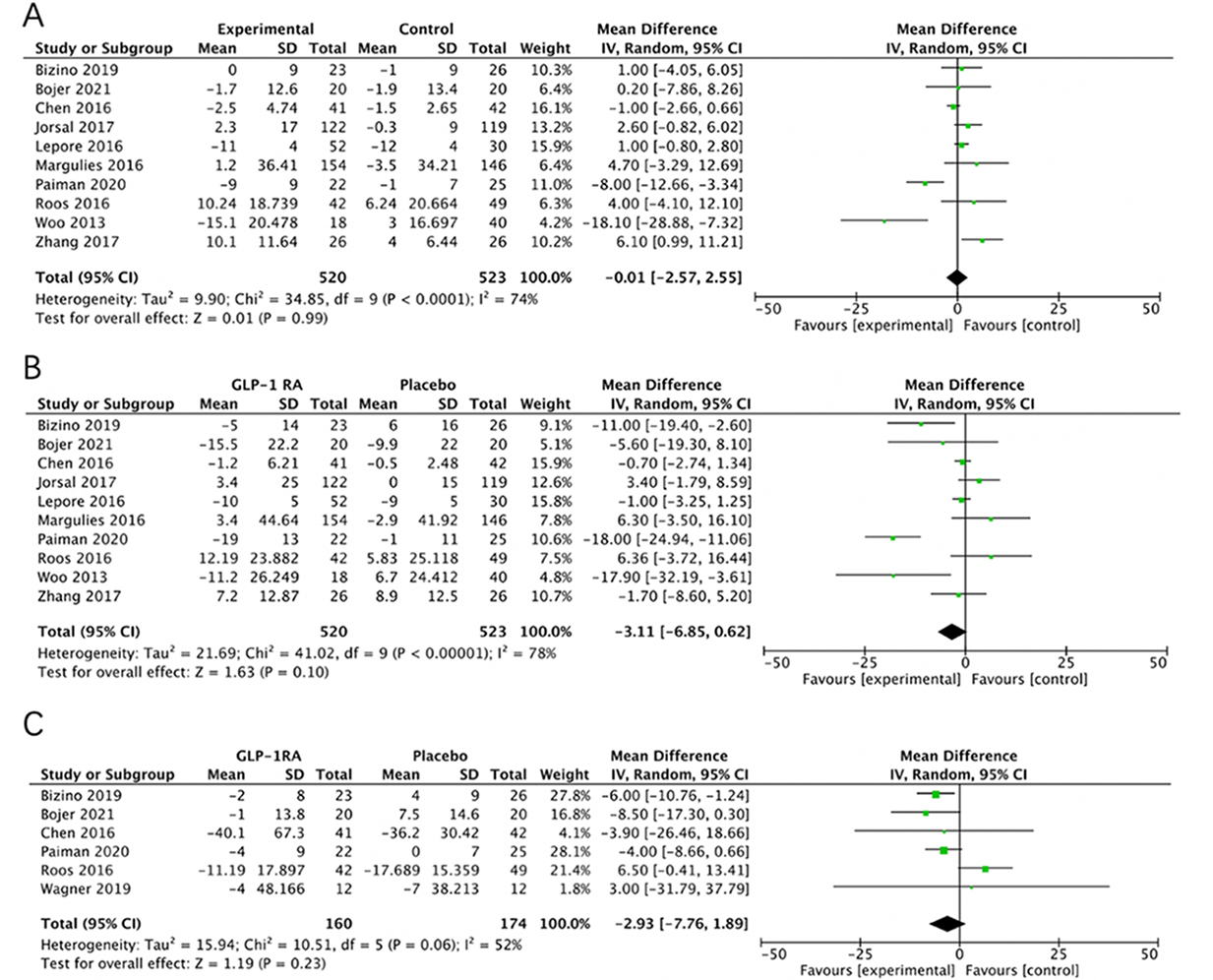
Figure 3.
3.3. Effect of GLP-1RAs on Left Ventricular Function
3.3.1. Systolic Function
The assessment of systolic function encompassed parameters such as left ventricular ejection fraction (LVEF), stroke volume (SV), and global longitudinal strain (GLS) (Figure 4A-4C). Thirteen studies [23-30, 32, 34-37], involving a total of 567 HF patients receiving GLP-1RAs and 566 HF patients receiving placebo, demonstrated a negligible improvement in LVEF measured through echocardiography, and the findings were not statistically significant (MD = -0.98; 95% CI:-2.08 to 0.12; P = 0.08; I 2= 91%) (Figure 4A). Furthermore, GLP-1RAs exhibited no observable effect on other systolic function parameters. An analysis of five studies [23, 24, 34, 37, 38] involving 117 GLP-1RA patients and 125 placebo patients, revealed a negligible difference in SV between the two groups (MD = -2.22; 95% CI: -9.04 to 4.60; P = 0.52; I 2= 83%) (Figure 4B). GLS, an emerging echocardiographic marker for assessing cardiac function in HF, showed no difference across six studies [24,25,28,32,36,38], involving 206 GLP-1RA patients and 226 placebo patients (MD = -0.29; 95% CI: -0.81 to 0.23; P = 0.27; I 2= 88%) (Figure 4C).

Figure 4.
3.3.2. Effect of GLP-1RAs on Diastolic Function
Diastolic function was assessed through the analysis of the early to late diastolic filling velocity ratio (E/A ratio) and the ratio of early transmitral filling velocity to early diastolic mitral annular velocity (E/e’ ratio) (Figure 5A-5B). An analysis of seven studies [23, 29, 33, 34, 36-38] comprising144 patients receiving GLP-1RAs and 170 patients receiving placebo, revealed that the E/A ratio was significantly improved in the GLP-1RA group compared to the placebo group (MD = -0.15; 95% CI: -0.21 to -0.09; P < 0.00001; I 2= 43%) (Figure 5A). Similarly, analysis of eight studies [23-25, 29, 31, 33, 36, 37] involving 350 HF patients treated with GLP-1RAs and 370 patients on placebo, demonstrated an improved E/e’ ratio within the GLP-1RA group compared to the placebo group (MD = -0.82; 95% CI: -1.53 to -0.11; P = 0.02; I 2= 62%) (Figure 5B).
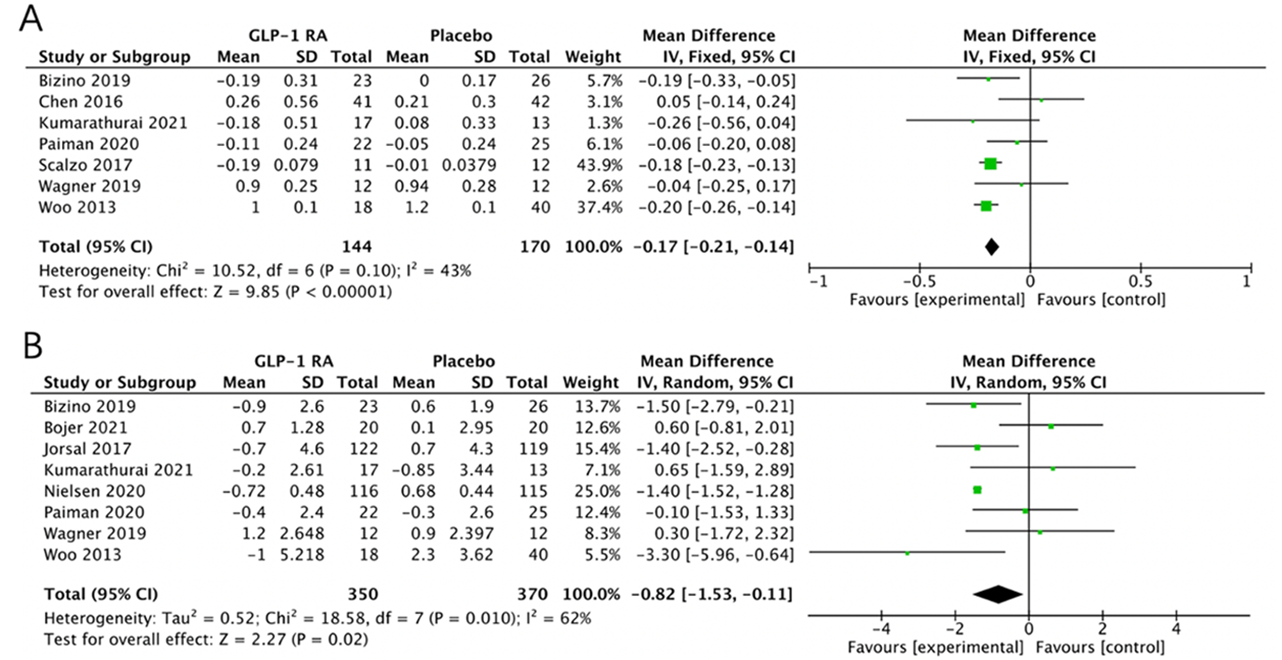
Figure 5.
4. Discussion
In this systematic review and meta-analysis, we enrolled a total of 1,417 patients from 16 randomized placebo-controlled trials to assess the effect of GLP-1RAs on cardiac structure and function in HF patients. Our findings revealed that GLP-1RAs confer beneficial effects on both the E/A ratio and E/e ratio, contributing to improved diastolic function. However, across the studies included in our analysis, GLP-1RAs did not demonstrate a significant improvement in systolic function or cardiac structures.
Diabetes and ischemia are recognized as independent risk factors for the development of HF [3]. Except for three studies (Jorsal 2017, Lepore 2016, Margulies 2016), a majority of the enrolled patients presented with comorbidities of diabetes and coronary artery disease. HFpEF patients exhibit impaired diastolic function, even when the ejection fraction exceeds 50%. Although HFpEF is associated with high morbidity and mortality, viable treatment modalities for this condition remain elusive. The prevalence of HFpEF has witnessed a notable increase in recent years, with epidemiological studies indicating that HFpEF accounts for more than 50% of all HF cases. In this current meta-analysis, GLP-1RAs exhibited beneficial effects on the diastolic function of HF patients, as evidenced by improved E/A and E/e′ ratios. Diastolic function refers to the ability of heart to fill during diastole, and its impairment is a significant hallmark of HFpEF. Many of the symptoms experienced by HFpEF patients, such as shortness of breath and fatigue, are related to impaired diastolic function. Improving diastolic function has the potential to mitigate these symptoms, thereby improving the patient's quality of life. Improved diastolic function among HFpEF patients may also result in fewer episodes of cardiac decompensation, consequently leading to a reduction in hospital admissions. Furthermore, although this meta-analysis does not directly illustrate the point, there exists a possibility that enhancing diastolic function could correlate with enhanced survival rates, similar to observations made in other HF treatments that target diastolic function. It is imperative to further explore the impact of GLP-1RAs on re-hospitalization rates and mortality. Given the increasing prevalence of HFpEF, these findings suggest that GLP-1RAs could emerge as a potential therapeutic option, particularly for patients with diabetes.
GLP-1 is an endogenous hormone secreted by intestinal endocrine cells. Accumulating evidence has demonstrated that GLP-1 exerts cardiac benefits beyond its metabolic effects. Previous studies have shown that GLP-1 mimetics alleviate endoplasmic reticulum stress, regulate autophagy, and stimulate anti-inflammatory signaling, potentially playing a cardioprotective role [39]. Due to the relatively low expression of GLP-1 receptors in ventricular cardiomyocytes, the effects of GLP-1RAs on the ventricles are mainly indirect, driven by the positive modulation of inflammation, endothelial function, and glucose uptake [40, 41]. Moreover, basic research indicates that GLP-1RAs mitigate cardiac remodeling following myocardial infarction by modulating changes in the extracellular matrix [42]. Calcium overload is a defining characteristic of heart failure. GLP-1RA treatment modulates cytosolic Ca2+ concentrations by suppressing phosphorylation of the ryanodine receptor 2(RyR2) and preventing activation of calmodulin-dependent protein kinase II (CaMKII) [43]. An in vitro study demonstrated that GLP-1RAs inhibit the production of mitochondrial and intracellular reactive oxygen species (ROS) in methylglyoxal-treated H9C2 cells, underscoring their anti-oxidative stress capabilities. While current basic research provides insight into the cardioprotective mechanisms of GLP-1RAs, a more comprehensive understanding necessitates experimentation.
A previous meta-analysis of four studies involving HFrEF patients revealed that short-term GLP-1RAs infusion exhibited a modest effect on LVEF (+4.4%, 95% CI: 1.36 to 7.44), with no significant change in brain natriuretic peptide (NT-proBNP) levels [44]. However, our meta-analysis combined both HFpEF and HFrEF patients. Although the effect on LVEF displayed a trend towards improvement with GLP-1RAs, the findings were not significantly different (P = 0.08). Given the relatively small sample size of the enrolled studies, additional RCTs focusing on heart failure subgroups are warranted to investigate the effect of GLP-1RAs on distinct types of heart failure in future studies.
Sodium-glucose cotransporter 2 inhibitors (SGLT-2i) are anti-diabetic drugs that have also demonstrated clinical benefits in HF. To date, no study has directly compared SGLT-2i with GLP-1RA in terms of HF outcomes using a head-to-head design. Nevertheless, real-world data and estimates from the meta-analysis are available. A recent meta-analysis revealed a higher risk of HF-related hospitalisation among individuals receiving GLP-1RAs compared to SGLT-2i [45]. This data suggests that, in comparison to SGLT-2i, GLP-1RA might be less effective in both the prevention and treatment of HF. Given the emerging roles of drugs like SGLT-2i in HF management, conducting head-to-head trials comparing GLP-1RAs with such drugs could prove beneficial in establishing a clear hierarchy of effectiveness.
This study has several limitations. Firstly, one of the limitations stems from the heterogeneity observed in the included studies. For instance, different studies included patients with varying comorbidities. Furthermore, the types of GLP-1RAs administered and their modes of administration varied among the studies. Additionally, differences in the methods of assessing cardiac function using echocardiography or cardiac magnetic resonance could contribute to variations in results. Secondly, despite the included studies demonstrating positive effects on both systolic and diastolic functions, the relatively small sample size of these studies hampers the ability to draw evidence-based guideline recommendations for GLP-1RAs.
5. Conclusion
GLP-1RA drugs may improve diastolic function in HF patients, but they do not affect the systolic function or cardiac structure. Given the variability in HF types and patient comorbidities, the application of GLP-1RAs should be tailored based on specific HF types and associated comorbidities.
Acknowledgement: We express our sincere appreciation to the editors at Easy-editing Ltd. for their proofreading of this manuscript, ensuring its language accuracy. Additionally, we extend our gratitude to MyMajor Ltd. for their invaluable assistance in coordinating the project meeting, which greatly facilitated the successful completion of this manuscript.
Author contributions: Xinyu Zhang and Hongyuan Zhang were responsible for conducting the literature search, selection, and bias assessment. Xinyu Zhang performed data extraction and contributed to manuscript writing. Hongyuan Zhang designed the project.
Conflicts of interest: The authors declare that they have no competing interests. The authors bear full responsibility for the content and composition of the article. All authors critically reviewed and revised the manuscript. The final version of the manuscript has been approved for submission, and all authors have agreed to assume accountability for the accuracy and integrity of the work.
References
- Roth, G.A. ; Mensah, G.A. ; Johnson, C . O . et al. Global Burden of Cardiovascular Diseases and Risk Factors , 1990 -2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol., 2020 . 76 ( 25 ): p. 2982 - 3021 .
- Rodgers, J.L. ; Jones, J. ; Bolleddu, S. I. ; et al.,Cardiovascular Risks Associated with Gender and Aging . J. Cardiovasc. Dev. Dis. , 2019 , 6 ( 2 ). DOI: https://doi.org/10.3390/jcdd6020019
- Dunlay, S.M. ; Givertz, M.M. ; Aguilar, D. ; et al . Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update . Circulation , 2019 , 140 ( 7 ): e294 - e324 . DOI: https://doi.org/10.1161/CIR.0000000000000691
- Dei Cas, A. ; Fonarow, G.C. ; Gheorghiade, M. ; et al . Concomitant diabetes mellitus and heart failure . Curr. Probl. Cardiol. , 2015 , 40 ( 1 ): 7 - 43 . DOI: https://doi.org/10.1016/j.cpcardiol.2014.09.002
- Boonman-de Winter, L.J. ; Rutten, F.H. ; Cramer, M.J. ; et al . High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes . Diabetologia , 2012 , 55 ( 8 ): 2154 - 2162 . DOI: https://doi.org/10.1007/s00125-012-2579-0
- From, A.M. ; Leibson, C.L. ; Bursi, F. ; et al . Diabetes in heart failure: prevalence and impact on outcome in the population . Am. J. Med. , 2006 , 119 ( 7 ): 591 - 599 . DOI: https://doi.org/10.1016/j.amjmed.2006.05.024
- Nichols, G.A. ; Gullion, C.M. ; Koro, C.E. ; et al . The incidence of congestive heart failure in type 2 diabetes: an update . Diabetes Care , 2004 , 27 ( 8 ): 1879 - 1884 . DOI: https://doi.org/10.2337/diacare.27.8.1879
- McMurray, J.J. ; Packer, M. ; Desai, A.S. ; et al . Angiotensin-neprilysin inhibition versus enalapril in heart failure . N. Engl. J. Med. , 2014 , 371 ( 11 ): 993 - 1004 . DOI: https://doi.org/10.1056/NEJMoa1409077
- Ponikowski, P. ; Voors, A.A. ; Anker, S.D. ; et al . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) . Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. , 2016 , 18 ( 8 ): 891 - 975 . DOI: https://doi.org/10.1002/ejhf.592
- Yancy, C.W. ; Jessup, M. ; Bozkurt, B. ; et al . 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America . J. Am. Coll. Cardiol. , 2017 , 70 ( 6 ): 776 - 803 . DOI: https://doi.org/10.1161/CIR.0000000000000509
- Packer, M. ; Anker, S.D. ; Butler, J. ; et al . Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure . N. Engl. J. Med. , 2020 , 383 ( 15 ): 1413 - 1424 .
- McMurray, J . J .V.; Solomon S.D. ; Inzucchi S.E.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med., 2019 , 381 ( 21 ): 1995 - 2008 .
- Zannad, F. ; Ferreira, J.P. ; Pocock, S.J. ; et al . SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials . Lancet , 2020 , 396 ( 10254 ): 819 - 829 . DOI: https://doi.org/10.1016/S0140-6736(20)31824-9
- Armstrong, P.W. ; Pieske, B. ; Anstrom, K.J. ; et al . Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction . N. Engl. J. Med. , 2020 , 382 ( 20 ): 1883 - 1893 . DOI: https://doi.org/10.1056/NEJMoa1915928
- Teerlink, J.R. ; Diaz, R. ; Felker, G.M. ; et al . Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure . N. Engl. J. Med. , 2021 , 384 ( 2 ): 105 - 116 .
- Marx, N. ; Husain, M. ; Lehrke, M. ; et al . GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients With Type 2 Diabetes . Circulation , 2022 , 146 ( 24 ): 1882 - 1894 . DOI: https://doi.org/10.1161/CIRCULATIONAHA.122.059595
- Cosentino, F. ; Grant, P.J. ; Aboyans, V. ; et al . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD . Eur. Heart J. , 2020 , 41 ( 2 ): 255 - 323 .
- Giugliano, D. ; Scappaticcio, L. ; Longo, M. ; et al . GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs . Cardiovasc. Diabetol. , 2021 , 20 ( 1 ): 189 . DOI: https://doi.org/10.1186/s12933-021-01366-8
- Palmer, S.C. ; Tendal, B. ; Mustafa, R.A. ; et al . Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials . B.M.J. , 2021 , 372 : m4573 .
- Natali, A. ; Nesti, L. ; Tricò, D. ; et al . Effects of GLP-1 receptor agonists and SGLT-2 inhibitors on cardiac structure and function: a narrative review of clinical evidence . Cardiovasc. Diabetol. , 2021 , 20 ( 1 ): 196 . DOI: https://doi.org/10.1186/s12933-021-01385-5
- Wong, S.Y. ; Lee, A . R .Y.B.; Sia A . H .J.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA) on Cardiac Structure and Function: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Cardiovasc. Drugs Ther., 2022 . DOI: https://doi.org/10.1007/s10557-022-07360-w
- Page, M.J. ; McKenzie, J.E. ; Bossuyt, P.M. ; et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . B.M.J. , 2021 , 372 : n71 .
- Bizino, M.B. ; Jazet, I.M. ; Westenberg, J . J .M.; et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol . 2019 Apr 30 ; 18 ( 1 ): 55 . doi: 10.1186/s12933-019-0857-6. Erratum in: Cardiovasc. Diabetol. , 2019 , 18 ( 1 ): 101 . DOI: https://doi.org/10.1186/s12933-019-0857-6
- Bojer, A.S. ; Sørensen, M.H. ; Bjerre, J. ; et al . Metabolic improvement with short-term, glucagon-like peptide-1 receptor agonist treatment does not improve cardiac diastolic dysfunction in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial . Diabetes Obes. Metab. , 2021 , 23 ( 10 ): 2374 - 2384 . DOI: https://doi.org/10.1111/dom.14480
- Jorsal, A. ; Kistorp, C. ; Holmager, P. ; et al.Effect of liraglutide, glucagon-like peptide-1 analogue, a ,on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre,double-blind,randomised, trial, placebo-controlled . Eur . J. Heart Fail. , 2017 , 19 ( 1 ): 69 - 77 . DOI: https://doi.org/10.1002/ejhf.657
- Lepore, J.J. ; Olson, E. ; Demopoulos, L. ; et al . Effects of the Novel Long-Acting GLP-1 Agonist, Albiglutide, on Cardiac Function, Cardiac Metabolism, and Exercise Capacity in Patients With Chronic Heart Failure and Reduced Ejection Fraction . J.A.C.C. Heart Fail. , 2016 , 4 ( 7 ): 559 - 566 . DOI: https://doi.org/10.1016/j.jchf.2016.01.008
- Margulies, K.B. ; Hernandez, A.F. ; Redfield, M.M. ; et al . Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial . J.A.M.A. , 2016 , 316 ( 5 ): 500 - 508 . DOI: https://doi.org/10.1001/jama.2016.10260
- Nielsen, R. ; Jorsal, A. ; Iversen, P. ; et al . Effect of liraglutide on myocardial glucose uptake and blood flow in stable chronic heart failure patients: A double-blind, randomized, placebo-controlled LIVE sub-study . J. Nucl. Cardiol. , 2019 , 26 ( 2 ): 585 - 597 . DOI: https://doi.org/10.1007/s12350-017-1000-2
- Wägner, A.M. ; Miranda-Calderín, G. ; Ugarte-Lopetegui, M . A,; et al. Effect of liraglutide on physical performance in type 2 diabetes: Results of a randomized, double-blind, controlled trial (LIPER2). Diabetes. Metab. , 2019 , 45 ( 3 ): 268 - 275 . DOI: https://doi.org/10.1016/j.diabet.2018.08.010
- Zhang, J.Y. ; Wang, X.Y. ; Wang, X . Effects of liraglutide on hemodynamic parameters in patients with heart failure . Oncotarget , 2017 , 8 ( 37 ): 62693 - 62702 . DOI: https://doi.org/10.18632/oncotarget.18570
- Nielsen, R. ; Jorsal, A. ; Tougaard, R.S. ; et al . The impact of the glucagon-like peptide-1 receptor agonist liraglutide on natriuretic peptides in heart failure patients with reduced ejection fraction with and without type 2 diabetes . Diabetes Obes. Metab. , 2020 , 22 ( 11 ): 2141 - 2150 . DOI: https://doi.org/10.1111/dom.14135
- Kumarathurai, P. ; Anholm, C. ; Nielsen, O.W. ; et al . Effects of the glucagon-like peptide-1 receptor agonist liraglutide on systolic function in patients with coronary artery disease and type 2 diabetes: a randomized double-blind placebo-controlled crossover study . Cardiovasc. Diabetol. , 2016 , 15 ( 1 ): 105 . DOI: https://doi.org/10.1186/s12933-016-0425-2
- Kumarathurai, P. ; Sajadieh, A. ; Anholm, C. ; et al . Effects of liraglutide on diastolic function parameters in patients with type 2 diabetes and coronary artery disease: a randomized crossover study . Cardiovasc. Diabetol. , 2021 , 20 ( 1 ): 12 . DOI: https://doi.org/10.1186/s12933-020-01205-2
- Chen, W.R. ; Shen, X.Q. ; Zhang, Y. ; et al . Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction . Endocrine , 2016 , 52 ( 3 ): 516 - 526 . DOI: https://doi.org/10.1007/s12020-015-0798-0
- Roos, S.T. ; Timmers, L. ; Biesbroek, P.S. ; et al . No benefit of additional treatment with exenatide in patients with an acute myocardial infarction . Int. J. Cardiol. , 2016 , 220 : 809 - 8114 . DOI: https://doi.org/10.1016/j.ijcard.2016.06.283
- Woo, J.S. ; Kim, W. ; Ha, S.J. ; et al . Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study . Arterioscler. Thromb. Vasc. Biol. , 2013 , 33 ( 9 ): 2252 - 2260 . DOI: https://doi.org/10.1161/ATVBAHA.113.301586
- Paiman, E . H .M.; van Eyk H.J. ; van Aalst M. M .A.; et al. Effect of Liraglutide on Cardiovascular Function and Myocardial Tissue Characteristics in Type 2 Diabetes Patients of South Asian Descent Living in the Netherlands: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Magn. Reson. Imaging, 2020 , 51 ( 6 ): 1679 - 1688 . DOI: https://doi.org/10.1002/jmri.27009
- Scalzo, R.L. ; Moreau, K.L. ; Ozemek, C. ; et al . Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes . J. Diabetes Complications , 2017 , 31 ( 2 ): 449 - 455 . DOI: https://doi.org/10.1016/j.jdiacomp.2016.10.003
- Rowlands, J. ; Heng, J. ; Newsholme, P. ; et al . Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function . Front. Endocrinol (Lausanne). , 2018 , 9 : 672 . DOI: https://doi.org/10.3389/fendo.2018.00672
- Drucker, D . J . The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. , 2016 , 24 ( 1 ): 15 - 30 . DOI: https://doi.org/10.1016/j.cmet.2016.06.009
- Baggio, L.L. ; Yusta, B. ; Mulvihill, E.E. ; et al . GLP-1 Receptor Expression Within the Human Heart . Endocrinology , 2018 , 159 ( 4 ): 1570 - 1584 . DOI: https://doi.org/10.1210/en.2018-00004
- Robinson, E. ; Cassidy, R.S. ; Tate, M. ; et al . Exendin-4 protects against post-myocardial infarction remodelling via specific actions on inflammation and the extracellular matrix . Basic Res. Cardiol. , 2015 , 110 ( 2 ): 20 . DOI: https://doi.org/10.1007/s00395-015-0476-7
- Chen, J. ; Xu, S. ; Zhou, W. ; et al . Exendin-4 Reduces Ventricular Arrhythmia Activity and Calcium Sparks-Mediated Sarcoplasmic Reticulum Ca Leak in Rats with Heart Failure . Int. Heart J. , 2020 , 61 ( 1 ): 145 - 152 . DOI: https://doi.org/10.1536/ihj.19-327
- Munaf, M. ; Pellicori, P. ; Allgar, V. ; et al . A meta-analysis of the therapeutic effects of glucagon-like Peptide-1 agonist in heart failure . Int. J. Pept. , 2012 , 2012 : 249827 . DOI: https://doi.org/10.1155/2012/249827
- McKee, A. ; Al-Khazaali, A. ; Albert, S . G . Glucagon-like Peptide-1 Receptor Agonists versus Sodium-Glucose Cotransporter Inhibitors for Treatment of T2DM. J. Endocr. Soc. , 2020 , 4 ( 5 ): bvaa037 . DOI: https://doi.org/10.1210/jendso/bvaa037






