Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Article
The Mechanism of Triacetyl Andrographolide in Inhibiting Proliferation of Pulmonary Artery Smooth Muscle Cells
Zhe Wang 1,†, Yi-Xuan Zhang 2,†, Jun-Zhuo Shi 1,†, Chen-Chen Wang 1, Meng-Qi Zhang 1, Yi Yan 3, Yan-Ran Wang 1, Lu-Ling Zhao 1, Jie-Jian Kou 4, Qing-Hui Zhao 5, Xin-Mei Xie 1, Yang-Yang He 1,2, Jun-Ke Song 6,*, Guang Han 1,7,*, and Xiao-Bin Pang 1,2,*
1 School of Pharmacy, Henan University, Kaifeng 475004, China
2 Department of Anesthesiology, Huaihe Hospital of Henan University, Kaifeng 475004, China
3 Heart Center and Shanghai Institute of Pediatric Congenital Heart Disease, Shanghai Children's Medical Center, National Children's Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai 200217, China
4 Department of Pharmacy, Huaihe Hospital of Henan University, Kaifeng 475004, China
5 Institute of Physical Culture, Huanghuai University, Zhumadian 463000, China
6 Beijing Key Laboratory of Drug Targets Identification and Drug Screening, Institute of Materia Medica, Chinese Academy of Medical Science and Peking Union Medical College, Beijing 100050, China
7 Henan Province Engineering Research Center of High Value Utilization to Natural Medical Resource in Yellow River Basin, Kaifeng 475004, China.
* Correspondence: smilejunke@imm.ac.cn (Jun-Ke Song); hang@henu.edu.cn (Guang Han); pxb@vip.henu.edu.cn (Xiao-Bin Pang)
† These authors contributed equally to this work.
Received: 17 April 2023
Accepted: 27 July 2023
Published: 28 September 2023
Abstract: This study examines the impact of triacetyl-diacyllactone (ADA) on the proliferation and migration of pulmonary artery smooth muscle cells (PASMCs) and elucidates its underlying mechanism. PASMCs derived from SD rats were cultured in vitro and randomly divided into four groups: control group, administration group, model group, and model administration group. The appropriate concentration of ADA for intervention was determined using the MTT assay. The proliferation ability of PASMCs in each group was assessed using the EdU assay. The migration ability of PASMCs in each group was evaluated using the Scratch wound healing assay and Transwell assay. Western blot analysis was performed to determine the protein expression levels of BMPR2, PCNA, and TGF-β1, as well as the phosphorylation levels of SMAD1 and SMAD2/3 in PASMCs from each group. Results show that at a concentration of 5 µmol/L, ADA did not impact the cell activity of PASMCs and instead exerted inhibitory effects on both the proliferation and migration of PASMCs induced by PDGF-BB. PDGF-BB was found to upregulate the expression levels of PCNA and TGF-β1, while downregulating the expression of BMPR2. Furthermore, PDGF-BB led to enhanced protein phosphorylation of SMAD1 and SMAD2/3. However, following ADA intervention, the expression levels of PCNA and TGF-β1 decreased, while the expression of BMPR2 increased. Additionally, protein phosphorylation of SMAD1 and SMAD2/3 decreased. Therefore, ADA can hinder the proliferation and migration of PASMCs induced by PDGF-BB, as well as suppress the upregulation of PCNA and TGF-β1 caused by PDGF-BB. Furthermore, the downregulation of BMPR2 may be associated with the inhibition of SMAD1 and SMAD2/3 signaling pathways.
Keywords:
Andrographolide proliferation pulmonary artery smooth muscle cells migration1. Introduction
Pulmonary arterial hypertension (PAH) is a relatively rare and life-threatening disease, characterized by endothelial dysfunction and muscularization of the vessel wall. This leads to pulmonary vascular remodeling, elevated pulmonary arterial pressure, and ultimately right ventricular heart failure [1, 2]. The diagnostic criteria for pulmonary hypertension has recently been revised to >20 mmHg, which is a reduction from the previous threshold of ≥25 mmHg [3]. Endothelial dysfunction causes apoptosis, muscularization, and loss of endothelial cell barrier function. Consequently, certain cytokines in conduit vessels induce abnormal proliferation of pulmonary artery smooth muscle cells (PASMCs) following the disruption of endothelial cell barrier function [4-6]. Numerous studies have highlighted the pivotal role of abnormal PASMC proliferation in the remodeling of pulmonary arterial vasculature and the progression of PAH [7, 8]. Epigenetic and metabolic regulatory mechanisms have been identified as key factors contributing to PAH [9, 10]. Given the complex pathological mechanisms involving genetic, epigenetic, metabolic abnormalities, hypoxia, and inflammatory responses, there is a scarcity of effective treatments for PAH [11, 12]. Currently, available drugs primarily target the reduction of pulmonary hypertension by inducing vasodilation [13]. However, there is a limited number of drugs that specifically inhibit abnormal PASMC proliferation to treat PAH. Recently, the emergence of Sotatercept has shown the potential in reducing pulmonary vascular resistance by modulating the signaling imbalance between growth-promoting and growth-inhibiting pathways [14]. Nevertheless, there remains a lack of clinical drugs capable of reversing vascular remodeling by inhibiting abnormal PASMC proliferation.
Andrographis paniculata is a widely used traditional Chinese medicine (TCM); it is known for its anti-inflammatory and antiviral properties [15, 16]. Andrographolide (AG), a diterpenoid extracted from Andrographis paniculata, is a major bioactive component of the plant. It has demonstrated anti-inflammatory and antitumor effects [17-19]. Its complex structure includes a twisted g-lactone ring, a double bond bridge D12, 13 linked to the g-lactone ring, an E-configured decahydronaphthalene ring, an exocyclic double bond, and three hydroxy groups [18]. Andrographolide can be chemically modified to generate new derivatives, offering the potential for the development of novel drug structures. In this study, we focused on a derivative of andrographolide called triacetyl andrographolide (ADA), which involves the addition of three acetyl groups to the parent ring, as well as another derivative known as Isoandrographolide (IA). Given the importance of abnormal PASMC proliferation in the development of PAH and the documented inhibitory effects of andrographolide on cancer cell proliferation [17, 20, 21], we investigated the inhibitory effects of andrographolide and its derivatives (ADA and IA) on abnormal PASMC proliferation. Therefore, this study aimed compare the effects of AG, ADA, and IA on PASMC activity.
Bone morphogenetic protein receptor type 2 (BMPR2), a type II receptor of the TGF-β cell signaling superfamily, plays a crucial role in the pathogenesis of PAH [22]. The reduction of BMPR2 has been linked to the inhibition of Smad1 signaling, which promotes the proliferation of PASMCs [23]. In this study, we examined the impact of ADA on the BMPR2/Smad1 pathway and further explored its inhibitory effects on PASMCs. Additionally, transforming growth factor β (TGF-β) and its downstream signaling molecule, SMAD, also contribute significantly to pulmonary vascular remodeling [24].
2. Materials and Methods
2.1. Materials
Fetal bovine serum (FBS) and Dulbecco's modified Eagle medium (DMEM) were procured from Gibco (USA). Anti-alpha smooth muscle actin antibody and anti-PCNA antibody were obtained from Abcam (Britain). BMPR2 polyclonal antibody, anti-GAPDH, and CoraLite594-conjugated Goat Anti-Mouse IgG (H+L) were purchased from Proteintech (China). Smad1 and p-smad1 antibodies were obtained from Cell Signaling Technology (USA). 5-ethynyl-2'-deoxyuridine (EdU) was obtained from RiboBio (China). FITC-phalloidin, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide), 4',6-diamidino-2-phenylindole (DAPI), BCA Protein Assay Kit, RIPA buffer, Protease Inhibitor Cocktail, and Phosphatase Inhibitor Cocktail were provided by Solarbio (China). Crystal violet was purchased from Beyotime (China). PDGF-BB was obtained from R&D Systems (USA). PVDF membranes were purchased from Millipore (USA). Andrographolide (AG), triacetyl andrographolide (ADA), and isoandrographolide (IA) were supplied by Han Guang Research Group, Henan University of Pharmacy.
2.2. Extraction of Rat Primary PASMCs
SD rats aged between three and eight weeks were humanely sacrificed through cervical dislocation and subsequently disinfected using 75% alcohol. By opening the pleural cavity, the aortic arch was exposed, revealing the "Y"-shaped pulmonary artery beneath it. The pulmonary artery was carefully excised and rinsed in PBS supplemented with 1% penicillin/streptomycin to remove connective tissue. The stripped blood vessels were then cut into small tissue blocks measuring 1 mm × 1 mm × 1 mm using ophthalmic scissors. These tissue blocks were evenly distributed on the dish's surface. Once dried, 6 mL of DMEM containing 20% FBS and 1% penicillin/streptomycin was gently added to the dish, and the cells were incubated at 37 °C in a 5% CO2 cell incubator [25]. The study was conducted according to the guidelines of the Declaration of Hel-sinki, and approved by Ethics Committee of Henan University (Approval No. HUSOM2021–070, Feb. 22, 2021).
2.3. Culture of Rat PASMCs
After 3–5 days, a small population of cells will dissociate from the tissue mass. Over 8–10 days, cells exhibit a cyclic pattern of growth and decline before reaching confluence and being passaged through trypsinization. The cell culture medium was typically changed every 2–3 days to support cell growth. For the extraction of PASMCs, DMEM supplemented with 20% FBS was used, followed by 10% FBS for subsequent passages. Cells in generations 3–8, exhibiting robust growth, were selected for further experimental investigations.
2.4. Immunofluorescence Staining and Cytoskeleton Staining
Cells cultured on coverslips for three days were utilized for staining purposes. Following three rinses with PBS, the cells were fixed with 4% paraformaldehyde at room temperature for 30 min. After additional washing with PBS, the cells were permeabilized with 0.5% Triton X-100 for 10 min. Subsequently, the cells were incubated with a FITC-phalloidin working solution (1:200) in the dark at 37 °C for 30 min. After three washes with PBS, they were incubated with 5% BSA at 37 °C for 30 min. For immunostaining, the fixed cells were incubated overnight at 4 °C with a mouse monoclonal antibody against smooth muscle alpha-actin (1:400), followed by incubation with CoraLite594-conjugated Goat Anti-Mouse IgG(H+L) (1:50) in a dark environment for 30 min. After three PBS washes, the cells were stained with 4',6-diamino-2-phenylindole (DAPI) (1:50) for 10 min. Following three additional PBS washes, the coverslip was sealed and imaged using a confocal microscope.
2.5. Experimental Grouping
The cell model was categorized into two groups. One group was the 5% FBS model group, which comprised of the control group, a control dosed group, a 5% FBS model group, and 5% FBS model dosed group. The other group was the PDGF-BB model group [26], which consisted of the control group, the control dosed group, the PDGF-BB (20 ng/mL) model group, and the PDGF-BB (20 ng/mL) model dosed group.
2.6. MTT Assay
PASMCs were seeded at a density of 6 × 103 cells/well in 96-well plates. Once the cells reached 70%-80% confluence, they were cultured in DMEM with 0.5% FBS for 24 h to synchronize their cell cycle. Following cell cycle synchronization, the cells were preincubated with the respective drugs for 1 h and then subjected to stimulation with 5% FBS and PDGF-BB (20 ng/mL). After 24 h of incubation, 10 µL of MTT (5 mg/mL) was added to each well and incubated for 4 h. Subsequently, 100 µL of DMSO reagent was added to each well, and gentle shaking was performed for 10 min. The OD values were measured using a microplate reader at 570 nm.
2.7. Scratch Wound Healing Assay
PASMCs were seeded in 6-well plates at a density of 3×105 cells/well. When the cell density reached 90%, a vertical line was created in the center of each well using a 200 µL pipette tip, followed by gentle washing with PBS twice. The scratches were then photographed at 0 h, 24 h, and 48 h after drug treatment. Finally, the scratch area was analyzed using Image J software.
2.8. EdU Proliferation Assay
PASMCs were seeded at a density of 6 × 103 cells per well in 96-well plates. Once the cells reached 70% to 80% confluence, they were cultured in DMEM supplemented with 0.5% FBS for 24 h to synchronize the cell cycle. Following cell cycle synchronization, the cells were preincubated with the respective drugs for 1 hour and then exposed to 5% FBS and PDGF-BB (20 ng/mL) to induce cell modeling. After 24 h of culture, the cells from each group were treated with EdU reagent for 3 h, followed by three rinses with PBS and fixation with 4% paraformaldehyde for 30 min. After washing with PBS, PASMCs were permeabilized with 0.5% Triton X-100 at room temperature for 10 min. Subsequently, the cells were incubated with the Click-iT staining reaction solution for 30 min, resulting in red fluorescence in EdU-positive nuclei. The nuclei were then stained with Hoechst (1:100) for 30 min, producing blue fluorescence. The cells were washed three times with PBS and imaged using an inverted fluorescence microscope. The number of EdU-positive cells was quantified using ImageJ software.
2.9. Cell Migration Assay
PASMCs at a concentration of 5 × 104 cells/well were suspended in serum-free DMEM and seeded into the upper chamber of a Transwell chamber. Following cell seeding, serum-free DMEM was added to the upper chamber, while PDGF-BB was added according to the experimental groups. For the serum-induced proliferation model, the lower chamber of the control group was supplemented with DMEM containing 0.5% FBS, while the model group received DMEM with 5% FBS. In the PDGF-BB-induced proliferation model, the lower chamber was uniformly filled with DMEM containing 5% FBS. After 24 h of treatment, the remaining cells were removed using cotton swabs, followed by two washes with PBS. The cells were then fixed with 4% paraformaldehyde for 30 min. After two additional washes with PBS, the cells were stained with 0.1% crystal violet for 30 min. Excess crystal violet was washed off with running water, and the cells were air-dried and photographed under a microscope. The number of cells that had migrated through the membrane was counted in randomly selected fields.
2.10. Western Blotting for Protein Expression
PASMCs were lysed using RIPA lysis buffer supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail. The total protein concentration was determined using the BCA Protein Assay Kit. Equal amounts of protein were separated by 10% SDS-PAGE and transferred onto PVDF membranes. Subsequently, the membranes were blocked with 5% (w/v) non-fat milk for 2 h at room temperature, followed by overnight incubation with primary antibodies at 4°C. Afterward, the membranes were probed with horseradish peroxidase-conjugated secondary antibodies. Finally, the membranes were exposed to an ECL substrate for 2–5 min, and the protein bands were visualized using ImageJ software. The primary antibodies used in this study included anti-PCNA (1:1000), anti-BMPR2 (1:1000), anti-Smad1 (1:1000), anti-p-Smad1 (1:1000), and anti-GAPDH (1:5000).
2.11. Statistical Analysis
All data are reported as mean ±SEM. GraphPad Prism 8.0 software was used for statistical analysis. One-way ANOVA was used to compare multiple groups. A p-value less than 0.05 was considered statistically significant, indicating a significant difference between the groups.
3. Results
3.1. Morphological Identification and Results of Cells
After three days of primary cell extraction, a small number of PASMCs sprouted from the tissue block. These cells exhibited an elongated fusiform or triangular shape. By day eight, some cells had begun to fuse, forming a distinctive "peak-valley" morphology. α-SMA is a characteristic protein of smooth muscle cells. Immunostaining of the primary cells revealed red fluorescence from F594-labeled α-SMA, while the cytoskeleton displayed green filamentous fluorescence when stained with FITC-labeled Phalloidin. Additionally, the cell nuclei were stained with blue fluorescence using DAPI. Notably, PASMCs derived from rat pulmonary aorta exhibited a distinct pattern with red cord-like fibers filling the cytoplasm in a straight, uninterrupted arrangement, along with a fascicular arrangement of green filamentous cytoskeleton. The PASMCs isolated through the explant method from rat pulmonary aorta were further confirmed by immunofluorescence using an anti-alpha smooth muscle cell (α-SMA) antibody and FITC-labeled Phalloidin staining for cytoskeletal visualization (Figure 1).

Figure 1. Identification of primary smooth muscle cells. Identification of PASMCs by cell morphology, immunostaining and cytoskeletal staining.
3.2. Effect of Andrographolide and Its Derivatives on Cell Viability of PASMCs
Initially, MTT assay was used toe assess the the effects of the three drugs on the viability of PASMCs. Triacetyl andrographolide (ADA) demonstrated the most impact on PASMC viability, with IC50 values ranging from 1 to 10 µmol/L (Figure 2A). Andrographolide (AG) induced PASMC proliferation at low concentrations, while exhibiting weak inhibitory effects at 100 µmol/L (Figure 2B). Isoandrographolide (IA) did not affect PASMCs at concentrations up to 100 µmol/L, indicating no inhibition of PASMC proliferation (Figure 2C). Based on these findings, ADA was selected for further experiments due to its potent inhibition of PASMCs. Concentrations between 1 and 10 µmol/L were used for further screening based on the IC50 value of ADA, and it was observed that 5 µmol/L ADA had no impact on PASMCs (Figure 2D). Therefore, experiments were conducted using 5 µmol/L ADA in two proliferating cell models. PASMCs were first starved with 0.5% FBS DMEM for 24 h and then cultured with 5% FBS DMEM for 24 h, resulting in approximately 50% proliferation compared to normal PASMCs. PASMCs were also starved with 0.5% FBS DMEM for 24 h and subsequently cultured with PDGF-BB (20 ng/mL) for 24 h, leading to around 40% proliferation compared to normal PASMCs. Notably, 5 µmol/L ADA inhibited the proliferation of PASMCs in both models by approximately 20% to 40% (Figure 2E, 2F). Thus, a concentration of 5 µmol/L ADA was chosen for subsequent characterization experiments.
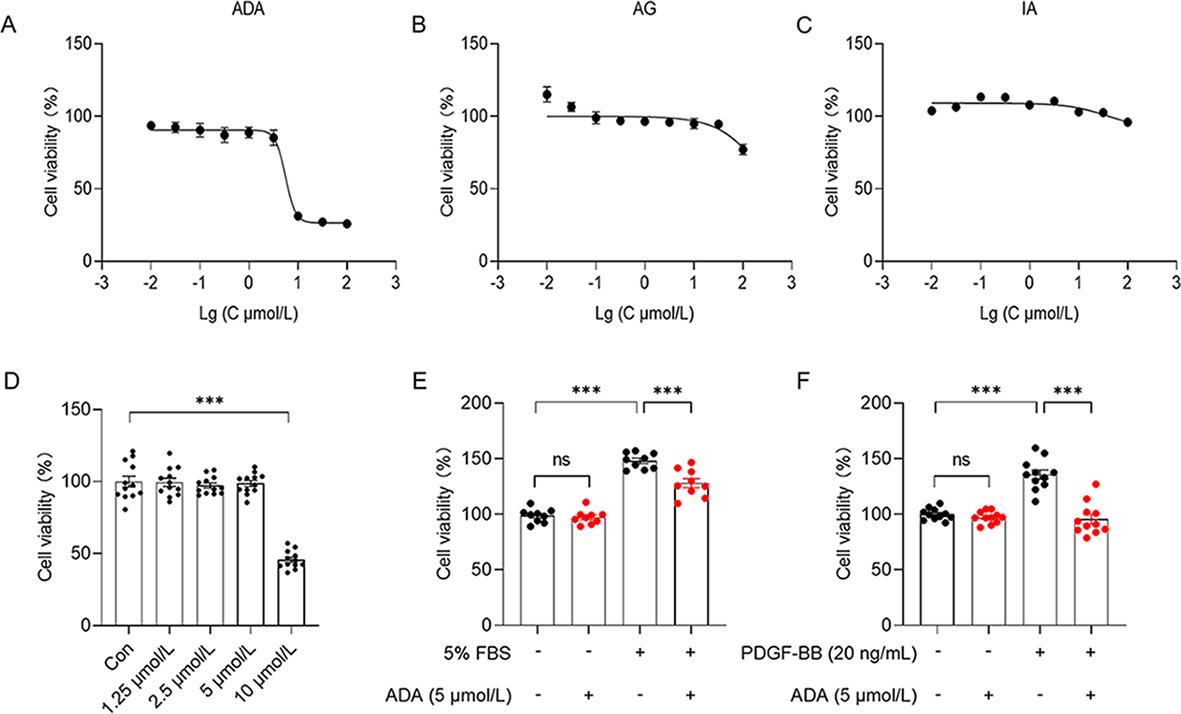
Figure 2. Concentration screening of the drugs performed using the MTT assay. The impact of ADA (n = 6) (A), AG (n = 6) (B), and IA (n = 6) (C) on the activity of normal PASMCs. The concentrations of ADA inhibiting normal PASMCs screened further (n = 12) (D). It was observed that ADA at a concentration of 5 µmol/L significantly inhibited the proliferation of PASMCs in models induced by 5% FBS (n = 9) (E) and PDGF-BB (20 ng/mL) (n = 11) (F). The data presented are the mean ±SEM. ***P < 0.001.
3.3. Cell Proliferation Model Constructed by Serum Inhibition of ADA
There were no significant differences in the number of EdU-positive cells and the total cell volume between the control group and the control administration group. However, the model group showed a significantly higher number of EdU-positive cells and total cell volume compared to the control group, indicating the successful establishment of the proliferation model. In contrast, the model administration group exhibited a lower number of EdU-positive cells and total cell volume compared to the model group. These findings suggest that 5 µmol/L ADA has the ability to inhibit the cell proliferation model induced by 5% FBS (Figure 3A).
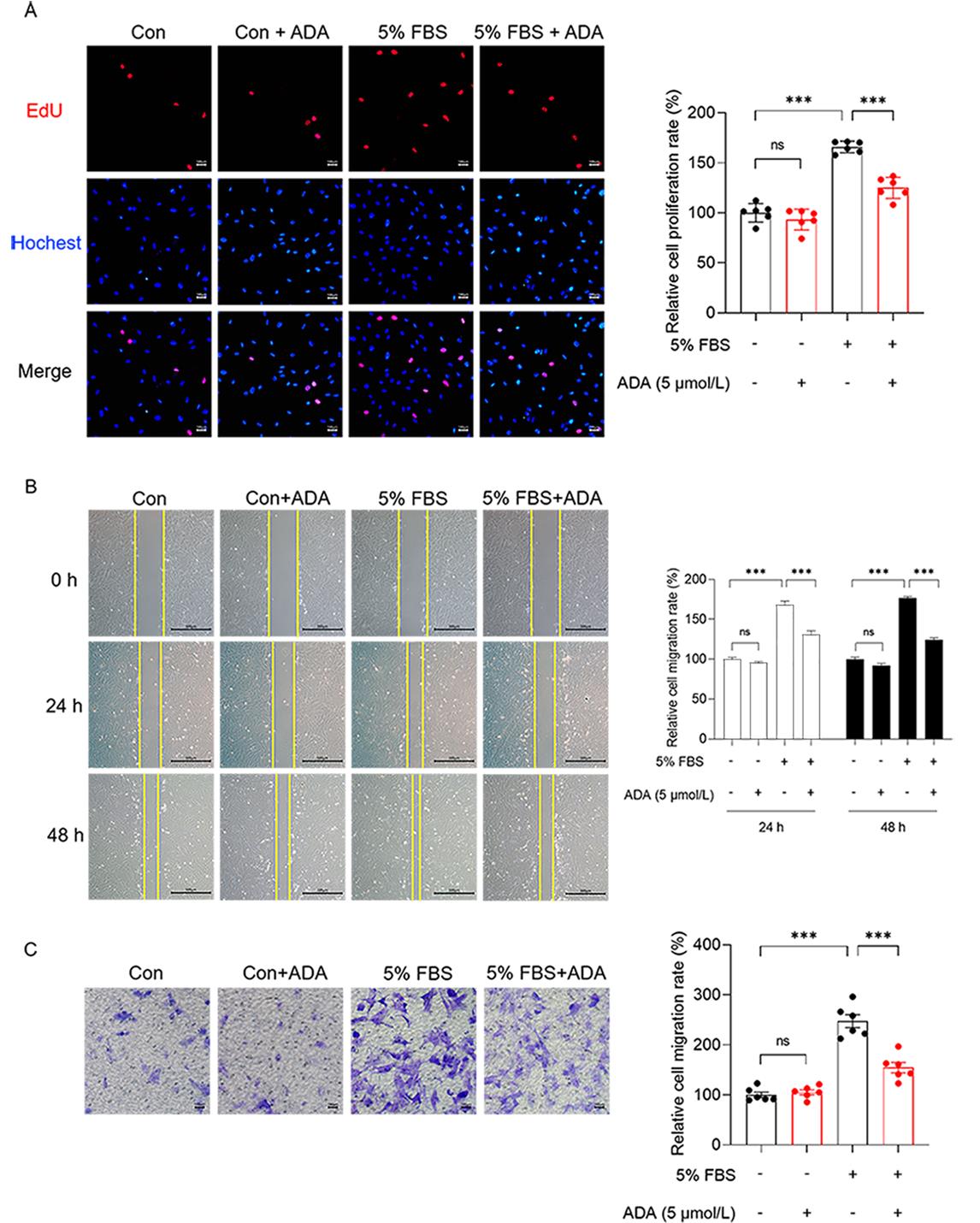
Figure 3 ADA at a concentration of 5 µmol/L inhibited the proliferation model of PASMCs induced by 5% FBS. A:The proliferation of PASMCs was assessed using the EdU assay (n = 6) . B and C:The extent of PASMCs proliferation was evaluated using the Scratch wound healing assay (n = 6) (B) and Transwell assay (n = 6) (C). All data are presented as the mean ±SEM. ***P < 0.001.
3.4. Cell Migration Model Constructed by Serum Inhibition of ADA
In regard to cell healing, the model group demonstrated a noticeable healing trend at 24 h compared to the other three groups. By 48 h, the model group had almost fully healed. This indicates the successful construction of the cell migration model using serum. The cell healing rate in the model administration group was slower than that in the model group (Figure 3B). In the Transwell cell migration experiment, a significantly higher number of cells passed through the compartments in the model group compared to the control group, which confirms serum-induced cell migration. However, the number of migrating cells was reduced in the model administration group compared to the model group (Figure 3C). These findings from the cell healing experiment and Transwell cell migration experiment demonstrate that 5 µmol/L ADA can inhibit the cell migration model induced by 5% FBS.
3.5. Cell Proliferation Model Constructed by PDGF-BB Inhibition of ADA
The ratio of EdU-positive cells to total cells between the control group and the control administration group was not significantly different. However, the model group exhibited a significantly higher number of EdU-positive cells and total cell volume compared to the control group, indicating the successful establishment of the proliferation model. In contrast, the model administration group showed a lower number of EdU-positive cells and total cell volume compared to the model group. These findings indicate that 5 µmol/L ADA can inhibit the cell proliferation model induced by PDGF-BB (20 ng/mL) (Figure 4A).

Figure 4. ADA at a concentration of 5 µmol/L inhibited the construction of the PASMCs proliferation model induced by PDGF-BB (20 ng/mL). A :The proliferation of PASMCs was analyzed using the EdU assay (n = 6). B and C : The extent of PASMCs proliferation was assessed using the Scratch Wound Healing assay (n = 6) (B) and the Transwell assay (n = 6) (C). All data are presented as the mean ±SEM. ***P < 0.001.
3.6. Cell Migration Model Constructed by PDGF-BB Inhibition of ADA
In the cell healing experiment, the model group demonstrated a distinct healing trend compared to the other three groups at 24 h, and nearly complete healing was observed in the model group at 48 h. This indicates PDGF-BB was able to induce the successful construction of the cell migration model. The cell healing rate in the model administration group was slower than that in the model group (Figure 4B). Furthermore, in the Transwell cell migration experiment, a significantly higher number of cells passed through the compartments in the model group compared to the control group, indicating the ability of PDGF-BB to induce cell migration. However, the number of migrating cells was reduced in the model administration group compared to the model group (Figure 4C). Based on the results of the cell healing and Transwell cell migration experiments, it can be concluded that 5 µmol/L ADA effectively inhibits the PDGF-BB-induced cell migration model.
3.7. The Expression Changes of BMPR2, PCNA, and p-SMAD1 in PASMCs induced by 5% FBS and PDGF-BB
Western blot analysis revealed that both PDGF-BB and 5% FBS increased the expression of PCNA in PASMCs. However, the administration of ADA reversed the increase in PCNA expression induced by PDGF-BB or 5% FBS (Figures 5A and 6A). In PASMCs stimulated with PDGF-BB or 5% FBS, the protein expression of BMPR2 decreased, while the level of p-SMAD1 increased (Figures 5B, 5C, 6B, and 6C). However, after treatment with ADA, the expression levels of BMPR2 increased, and the phosphorylation of SMAD1 decreased (Figures 5B and 6C). These findings suggest that ADA's inhibition of abnormal proliferation and migration of PASMCs induced by PDGF-BB or 5% FBS may be mediated through the BMPR2/Smad1 signaling pathway.
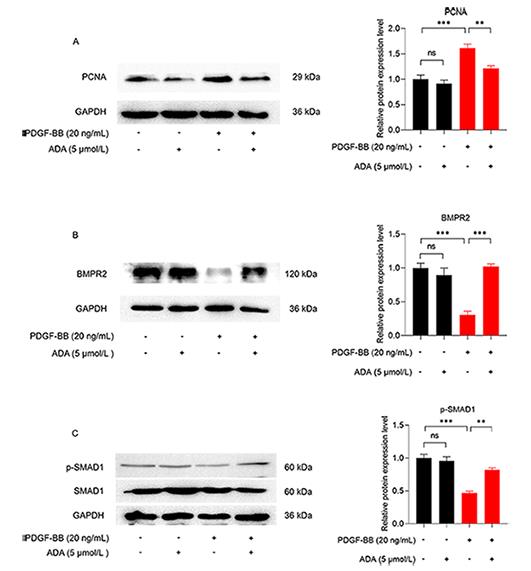
Figure 5. The expression levels of BMPR2, PCNA, and p-SMAD1 in PASMCs induced by PDGF-BB. A :The impact of ADA and PDGF-BB on PCNA expression. B and C The effects of ADA and PDGF-BB on the expression of BMPR2 and p-SMAD1. All data are presented as the mean ±SEM (n = 4). **P < 0.01, ***P < 0.001.
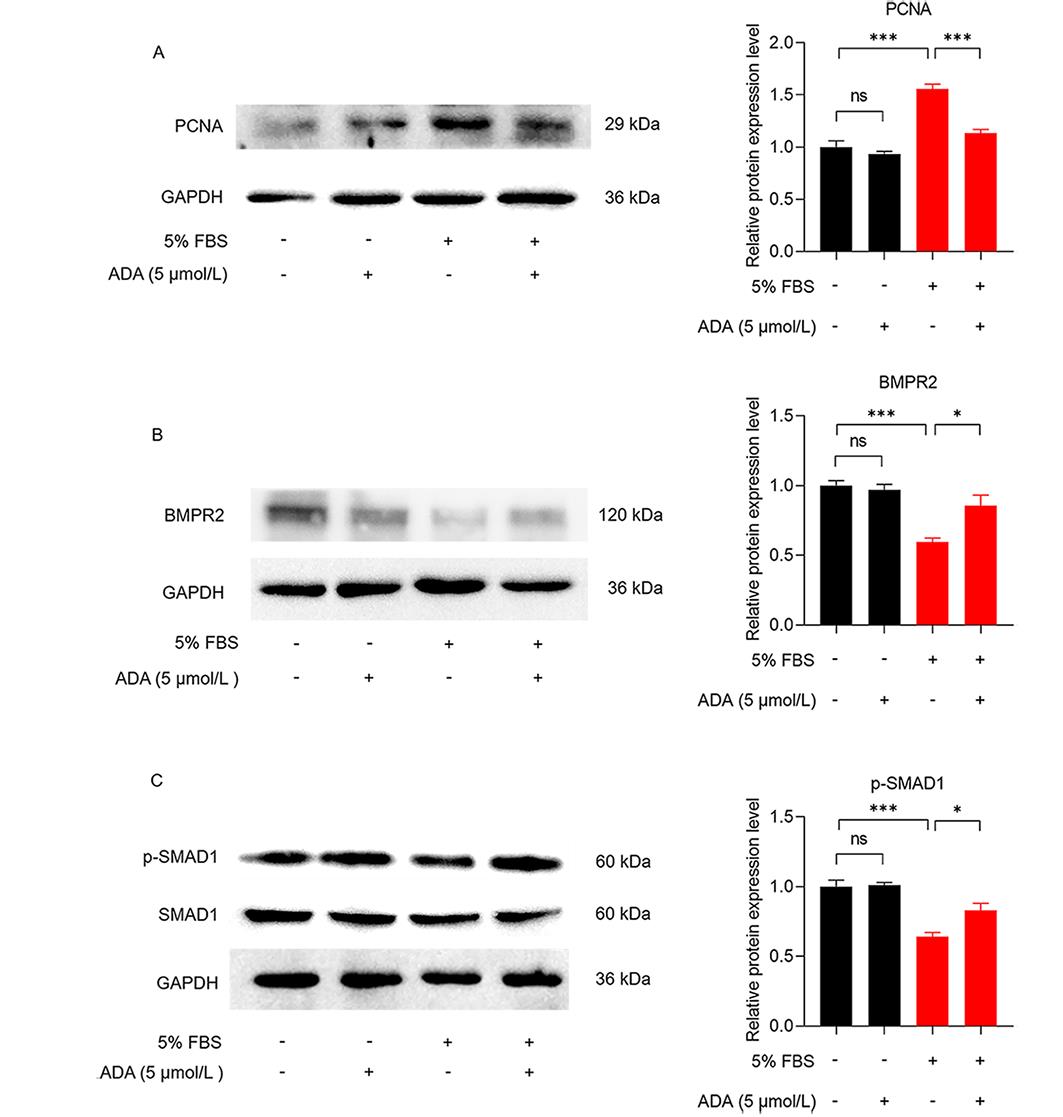
Figure 6. Changes in the expression of BMPR2, PCNA, and p-SMAD1 in PASMCs induced by 5% FBS. A:The effect of ADA and 5% FBS on PCNA expression. B and C:The effects of ADA and 5% FBS on the expression of BMPR2 and p-SMAD1. All data are presented as the mean ±SEM (n = 4). *P < 0.05, ***P < 0.001.
3.8. Expression Changes of TGF-β1 and p-SMAD2/3 in PASMCs induced by PDGF-BB.
Western blot analysis revealed that PDGF-BB upregulated the expression of TGF-β1 in PASMCs, while ADA treatment led to a decrease in TGF-β1 expression (Figure 7A). PDGF-BB also induced phosphorylation of SMAD2/3, a downstream signaling pathway of TGF-β1, whereas ADA treatment resulted in a decrease in SMAD2/3 phosphorylation (Figure 7B). These findings suggest that ADA may inhibit the proliferation and migration of PASMCs by downregulating the expression of TGF-β1 in PASMCs.

Figure 7. Changes in the expression of TGF- β1 and p-SMAD2/3 in PASMCs induced by PDGF-BB. A:Effect of ADA and PDGF-BB on TGF-β1 expression. B:Effects of ADA and PDGF-BB on p-SMAD2/3 expression. All data are presented as the mean ±SEM (n = 4). *P < 0.05, ***P < 0.001.
4. Discussion
Pulmonary blood vessels consist of fibroblasts in the outer layer; smooth muscle cells in the middle layer; and endothelial cells in the inner layer. Patients with pulmonary arterial hypertension (PAH) experience pathological changes in the pulmonary artery, which lead to dysfunction in endothelial cell production and loss of their protective effect on blood vessels. This loss of protection results in the abnormal proliferation of smooth muscle cells, leading to the formation of plexiform lesions [27]. PAH primarily arises from vascular remodeling of the pulmonary arterioles, causing vascular obstruction and elevated pulmonary arterial pressure [28]. Current research on PAH treatment focuses on reducing pulmonary arterial pressure by inhibiting the abnormal proliferation of smooth muscle cells to slow down vascular remodeling [29, 30]. Available drugs primarily alleviate pulmonary hypertension by vasodilation, thereby improving patients' quality of life [24]. However, these drugs do not address the increased pulmonary arterial pressure caused by pulmonary vascular remodeling through the inhibition of abnormal smooth muscle cell proliferation.
In recent years, andrographolide has been found to possess anticancer properties against liver cancer, lung cancer, breast cancer, and colon cancer [28, 31-33]. The abnormal proliferation of smooth muscle cells is similar to that of tumor cells, and since andrographolide has an inhibitory effect on tumor cells, it is worth exploring whether drugs derived from andrographolide, such as ADA and IA, have significant inhibitory effects on the proliferation of smooth muscle cells. In this study, we tested AG, IA, and ADA on normal smooth muscle cells, and only ADA showed an effect on their activity. This may be attributed to the structural differences between ADA and AG; ADA, with three acetyl groups added to its structure compared to AG, exhibited superior efficacy. After confirming the effect of ADA on normal smooth muscle cells, we determined a non-toxic concentration of ADA at 5 µmol/L through cell viability testing.
Next, we established a proliferating smooth muscle cell model using 5% FBS and PDGF-BB (20 ng/mL) [22, 34] to investigate whether 5 µmol/L ADA could inhibit the proliferation of this model. MTT assay confirmed that 5 µmol/L ADA inhibited the proliferation of both smooth muscle cell models. Therefore, we concluded that 5 µmol/L ADA can inhibit the abnormal proliferation of smooth muscle cells without affecting the viability of normal smooth muscle cells. After determining the appropriate dose of ADA, we further demonstrated its inhibitory effects on proliferation and migration of the serum- and PDGF-BB-induced smooth muscle cell proliferation model through EdU proliferation assay, scratch wound healing assay, and Cell Migration Assay.
In our investigation using Western blot analysis, we observed a significant increase in the proliferation marker PCNA in PASMCs induced by PDGF-BB (20 ng/mL). However, this increase was reversed when the PASMCs were treated with 5 µmol/L ADA. Previous studies have demonstrated the involvement of the BMPR2/Smad1 signaling pathway (Figure 6) in the progression of PAH [23]. We observed a notable decrease in the protein expression of BMPR2 and phosphorylated Smad1 in PDGF-BB-induced PASMCs, but treatment with 5 µmol/L ADA resulted in an increase in their expression levels, thus, inhibiting the proliferation of PASMCs. Based on these findings, it can be concluded that ADA may inhibit PASMC proliferation by modulating the BMPR2/Smad1 signaling pathway.
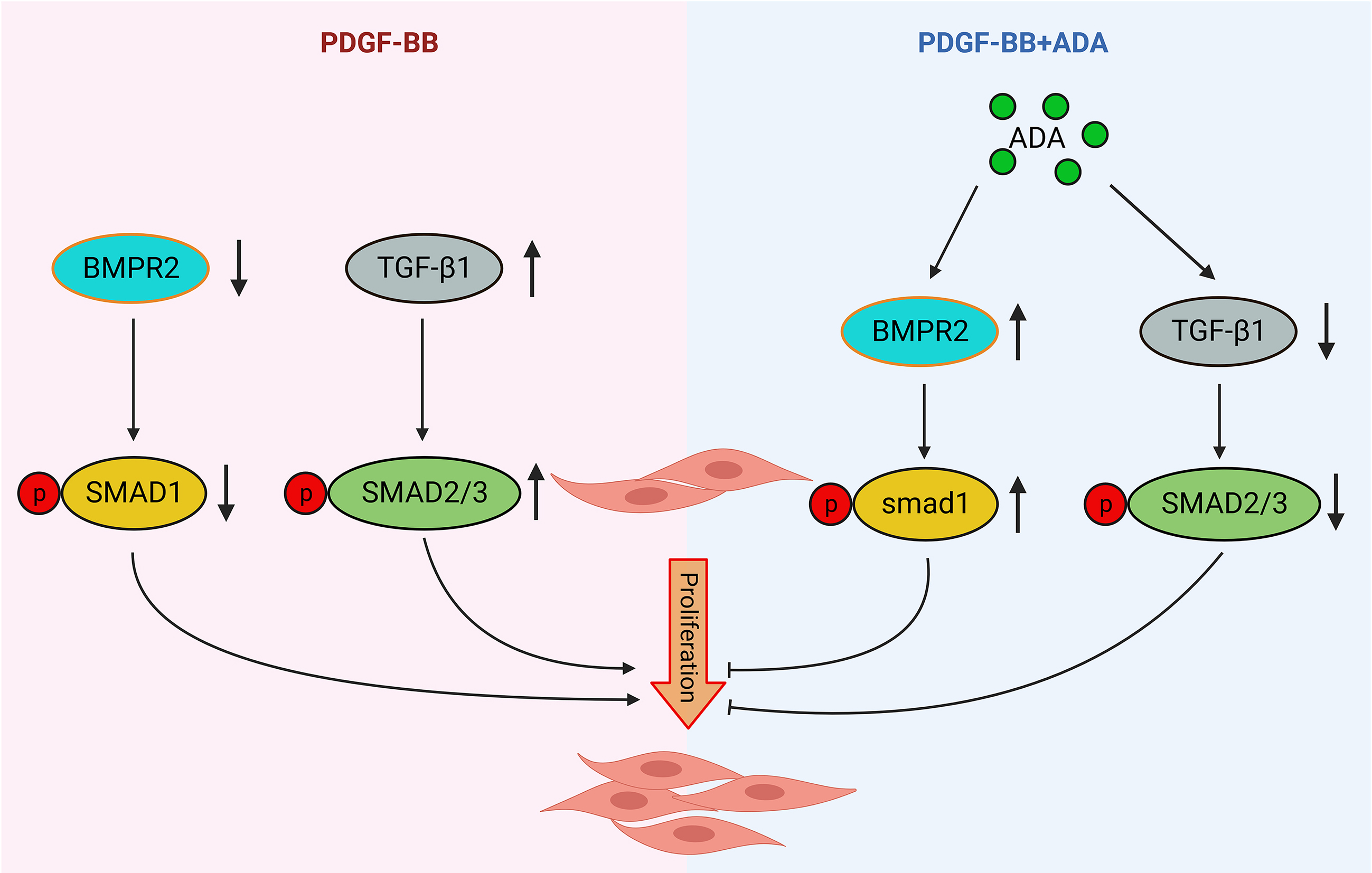
Figure 8. Mechanism of ADA-mediated inhibition of vascular smooth muscle cell proliferation through regulation of BMPR2 and TGF-β1.
5. Conclusion
In summary, our experimental findings demonstrate that ADA exhibited stronger inhibitory effects on PASMCs compared to AG and IA. ADA effectively suppressed the proliferation and migration of PASMCs in models induced by 5% FBS and PDGF-BB. These results suggest that ADA may counteract pulmonary artery vascular remodeling by modulating the BMPR2/Smad1 signaling pathway or inhibiting TGF-β1, ultimately leading to a reduction in pulmonary artery pressure. These findings provide a basis for exploring the therapeutic potential of natural drugs in the treatment of PAH.
Author Contributions: Zhe Wang, Jun-Zhuo Shi and Yi-Xuan Zhang drafted the manuscript and prepared the figures; Yi Yan, Chen-Chen Wang, Meng-Qi Zhang and Yan-Ran Wang participated in the writing of manuscripts and literature collection; Lu-Ling Zhao and Qing-Hui Zhao participated in the drawing of figures; Jie-Jian Kou participated in the literature collection and analysis; Yang-Yang He and Xin-Mei Xie participated in revision of manuscript; Jun-Ke Song, Guang Han and Xiao-Bin Pang proposed the concept, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding: This study was funded by the Projects of National Natural Science Foundation of China (82200065, 82170058, 82241007), Shanghai Pujiang Program (22PJ1410100), Young Talent Program of Shanghai Municipal Health Commission (2022YQ070), Science Foundation for Outstanding Young Scholars of Henan Province (212300410027), Joint Fund for Science and Technology R&D Plan of Henan Province (222103810055), Key Scientific Research Projects of Henan Higher Education Institutions (22A310002), Special Project for Key R&D and Promotion of Henan Province (232102311233) and Project of China Postdoctoral Science Foundation (2022M711051).
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Hel-sinki, and approved by Ethics Committee of Henan University (Approval No. HUSOM2021–070, Feb. 22, 2021).
Informed Consent Statement: Not applicable.
Conflicts of Interest: The authors declare that they have no competing interests.
References
- Lai, Y.C. ;Potoka, K.C. ;Champion, H.C. ; et al . Pulmonary arterial hypertension: the clinical syndrome . Circ. Res. , 2014 , 115 ( 1 ): 115 - 130 . DOI: https://doi.org/10.1161/CIRCRESAHA.115.301146
- Spiekerkoetter, E. ;Kawut, S.M. ;de Jesus Perez, V . A . New and Emerging Therapies for Pulmonary Arterial Hypertension. Annu. Rev. Med. , 2019 , 70 : 45 - 59 . DOI: https://doi.org/10.1146/annurev-med-041717-085955
- Humbert, M. ;Kovacs, G. ;Hoeper, M.M. ; et al . 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension . Eur. Heart J. , 2022 , 43 ( 38 ): 3618 - 3731 .
- Guignabert, C. ;Tu, L. ;Girerd, B. ; et al . New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication . Chest , 2015 , 147 ( 2 ): 529 - 537 . DOI: https://doi.org/10.1378/chest.14-0862
- Evans, C.E. ;Cober, N.D. ;Dai, Z. ; et al . Endothelial cells in the pathogenesis of pulmonary arterial hypertension . Eur. Respir. J. , 2021 , 58 ( 3 ): 2003957 . DOI: https://doi.org/10.1183/13993003.03957-2020
- He, Y.Y. ;Xie, X.M. ;Zhang, H.D. ; et al . Identification of Hypoxia Induced Metabolism Associated Genes in Pulmonary Hypertension . Front. Pharmacol. , 2021 , 12 : 753727 . DOI: https://doi.org/10.3389/fphar.2021.753727
- Tajsic, T. ;Morrell, N . W . Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr. Physiol. , 2011 , 1 ( 1 ): 295 - 317 . DOI: https://doi.org/10.1002/cphy.c100026
- Wang, S. ;Yan, Y. ;Xu, W.J. ; et al . The Role of Glutamine and Glutaminase in Pulmonary Hypertension . Front. Cardiovasc. Med. , 2022 , 9 : 838657 . DOI: https://doi.org/10.3389/fcvm.2022.838657
- Yan, Y. ;He, Y.Y. ;Jiang, X. ; et al . DNA methyltransferase 3B deficiency unveils a new pathological mechanism of pulmonary hypertension . Sci. Adv. , 2020 , 6 ( 50 ): eaba2470 . DOI: https://doi.org/10.1126/sciadv.aba2470
- He, Y.Y. ;Yan, Y. ;Jiang, X. ; et al . Spermine promotes pulmonary vascular remodelling and its synthase is a therapeutic target for pulmonary arterial hypertension . Eur. Respir. J. , 2020 , 56 ( 5 ): 2000522 . DOI: https://doi.org/10.1183/13993003.00522-2020
- He, Y.Y. ;Yan, Y. ;Chen, J.W. ; et al . Plasma metabolomics in the perioperative period of defect repair in patients with pulmonary arterial hypertension associated with congenital heart disease . Acta. Pharmacol. Sin. , 2022 , 43 ( 7 ): 1710 - 1720 . DOI: https://doi.org/10.1038/s41401-021-00804-3
- Wu, X.H. ;He, Y.Y. ;Chen, Z.R. ; et al . Single-cell analysis of peripheral blood from high-altitude pulmonary hypertension patients identifies a distinct monocyte phenotype . Nat. Commun. , 2023 , 14 ( 1 ): 1820 . DOI: https://doi.org/10.1038/s41467-023-37527-4
- Ruopp, N.F. ;Cockrill, B . A . Diagnosis and Treatment of Pulmonary Arterial Hypertension : A Review. J.A.M.A. , 2022 , 327 ( 14 ): 1379 - 1391 . DOI: https://doi.org/10.1001/jama.2022.4402
- Humbert, M. ;McLaughlin, V. ;Gibbs, J . S .R.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. , 2021 , 384 ( 13 ): 1204 - 1215 . DOI: https://doi.org/10.1056/NEJMoa2024277
- Jiang, M. ;Sheng, F. ;Zhang, Z. ; et al . Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents . J. Ethnopharmacol. , 2021 , 272 : 113954 . DOI: https://doi.org/10.1016/j.jep.2021.113954
- Chua, L . S . Review on liver inflammation and antiinflammatory activity of Andrographis paniculata for hepatoprotection. Phytother. Res. , 2014 , 28 ( 11 ): 1589 - 1598 . DOI: https://doi.org/10.1002/ptr.5193
- Peng, Y. ;Wang, Y. ;Tang, N. ; et al . Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway . J. Exp. Clin. Cancer Res. , 2018 , 37 ( 1 ): 248 . DOI: https://doi.org/10.1186/s13046-018-0926-9
- Dai, Y. ;Chen, S.R. ;Chai, L. ; et al . Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide . Crit. Rev. Food Sci. Nutr. , 2019 , 59 ( sup1 ): S17 - S29 . DOI: https://doi.org/10.1080/10408398.2018.1501657
- Burgos, R.A. ;Alarcón, P. ;Quiroga, J. ; et al . Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism . Molecules , 2020 , 26 ( 1 ): 5 . DOI: https://doi.org/10.3390/molecules26010005
- Tohkayomatee, R. ;Reabroi, S. ;Tungmunnithum, D. ; et al . Andrographolide Exhibits Anticancer Activity against Breast Cancer Cells (MCF-7 and MDA-MB-231 Cells) through Suppressing Cell Proliferation and Inducing Cell Apoptosis via Inactivation of ER-α Receptor and PI3K/AKT/mTOR Signaling . Molecules , 2022 , 27 ( 11 ): 3544 . DOI: https://doi.org/10.3390/molecules27113544
- Dai, L. ;Wang, G. ;Pan, W . Andrographolide Inhibits Proliferation and Metastasis of SGC7901 Gastric Cancer Cells . Biomed. Res. Int. , 2017 , 2017 : 6242103 . DOI: https://doi.org/10.1155/2017/6242103
- Bisserier, M. ;Mathiyalagan, P. ;Zhang, S. ; et al . Regulation of the Methylation and Expression Levels of the BMPR2 Gene by SIN3a as a Novel Therapeutic Mechanism in Pulmonary Arterial Hypertension . Circulation , 2021 , 144 ( 1 ): 52 - 73 . DOI: https://doi.org/10.1161/CIRCULATIONAHA.120.047978
- Zhang, Y. ;Peng, B. ;Han, Y . MiR-23a regulates the proliferation and migration of human pulmonary artery smooth muscle cells (HPASMCs) through targeting BMPR2/Smad1 signaling . Biomed Pharmacother , 2018 , 103 : 1279 - 1286 . DOI: https://doi.org/10.1016/j.biopha.2018.04.172
- Barberà, J.A. ;Román, A. ;Gómez-Sánchez, M.Á. ; et al . Guidelines on the Diagnosis and Treatment of Pulmonary Hypertension: Summary of Recommendations . Arch. Bronconeumol (Engl Ed). , 2018 , 54 ( 4 ): 205 - 215 . DOI: https://doi.org/10.1016/j.arbr.2017.11.017
- Peng, G. ;Xu, J. ;Liu, R. ; et al . Isolation, culture and identification of pulmonary arterial smooth muscle cells from rat distal pulmonary arteries . Cytotechnology , 2017 , 69 ( 5 ): 831 - 840 . DOI: https://doi.org/10.1007/s10616-017-0081-8
- Yan, Y. ;Jiang, R. ;Yuan, P. ; et al . Implication of proliferation gene biomarkers in pulmonary hypertension . Animal Model. Exp. Med. , 2021 , 4 ( 4 ): 369 - 380 . DOI: https://doi.org/10.1002/ame2.12191
- Woo, K.V. ;Shen, I.Y. ;Weinheimer, C.J. ; et al . Endothelial FGF signaling is protective in hypoxia-induced pulmonary hypertension . J. Clin. Invest. , 2021 , 131 ( 17 ): e141467 . DOI: https://doi.org/10.1172/JCI141467
- Langleben, D. ;Orfanos, S.E. ;Fox, B.D. ; et al . The Paradox of Pulmonary Vascular Resistance: Restoration of Pulmonary Capillary Recruitment as a Sine Qua Non for True Therapeutic Success in Pulmonary Arterial Hypertension . J. Clin. Med. , 2022 , 11 ( 15 ): 4568 . DOI: https://doi.org/10.3390/jcm11154568
- White, R.J. ;Meoli, D.F. ;Swarthout, R.F. ; et al . Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension . Am. J. Physiol. Lung Cell. Mol. Physiol. , 2007 , 293 ( 3 ): L583 - 90 . DOI: https://doi.org/10.1152/ajplung.00321.2006
- Fan, Y. ;Gu, X. ;Zhang, J. ; et al . TWIST1 Drives Smooth Muscle Cell Proliferation in Pulmonary Hypertension via Loss of GATA-6 and BMPR2 . Am. J. Respir. Crit. Care Med. , 2020 , 202 ( 9 ): 1283 - 1296 . DOI: https://doi.org/10.1164/rccm.201909-1884OC
- Jiaqi, L. ;Siqing, H. ;Qin, W. ; et al . Andrographolide promoted ferroptosis to repress the development of non-small cell lung cancer through activation of the mitochondrial dysfunction . Phytomedicine , 2023 , 109 : 154601 . DOI: https://doi.org/10.1016/j.phymed.2022.154601
- Chou, Y.J. ;Lin, C.C. ;Hsu, Y.C. ; et al . Andrographolide suppresses the malignancy of triple-negative breast cancer by reducing THOC1-promoted cancer stem cell characteristics . Biochem. Pharmacol. , 2022 , 206 : 115327 . DOI: https://doi.org/10.1016/j.bcp.2022.115327
- Janani, B. ;Vijayakumar, M. ;Priya, K. ; et al . A network-based pharmacological investigation to identify the mechanistic regulatory pathway of andrographolide against colorectal cancer . Front. Pharmacol. , 2022 , 13 : 967262 . DOI: https://doi.org/10.3389/fphar.2022.967262
- Lu, Q.B. ;Wan, M.Y. ;Wang, P.Y. ; et al . Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade . Redox. Biol. , 2018 , 14 : 656 - 668 . DOI: https://doi.org/10.1016/j.redox.2017.11.012






