Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
Pathophysiological Mechanisms and Pharmaceutical Interventions of Myocardial Infarction with Depression
Mingyang Xu 1, Yinxiang Wei 2, Zhenhui Wang 1, Yaohui Wang 2, Xiaoming Zhong 3,*, and Qiying Chen 4,*
1 School of medicine, Henan University, Kaifeng 475000, China.
2 Joint National Laboratory for Antibody Drug Engineering, Henan University, Kaifeng 475004, China.
3 Department of Cardiology, Huaihe Hospital of Henan University, Kaifeng 475000, China.
4 Department of Cardiology, Huashan Hospital, Fudan University, Shanghai 200040, China.
* Correspondence: Xiaoming Zhong (zxm10020202@126.com); Qiying Chen (chenqiying@huashan.org.cn)
Received: 23 March 2023
Accepted: 10 May 2023
Published: 27 June 2023
Abstract: The strong association between acute myocardial infarction (AMI) and major depression disorder(MDD)is a universally accepted. Patients with AMI complicated by MDD often have poor prognosis. Most early studies focused on how AMI leads to MDD, but there are few reports on depression-induced AMI. In terms of mechanism, inflammation, the hypothalamic-pituitary-adrenal axis (HPA axis) and brain-gut axis may be involved in the occurrence and development of MDD after AMI. The inflammatory injury, abnormal sympathetic and vagal nerve activity, HPA axis overactivation, overeating and some therapeutic medicine administration in patients with MDD can also be risk factors for AMI. Both behavioral and pharmaceutical interventions are important for the treatment of AMI with MDD. More drugs are being developed and tested. At present, there are still many issues, needing to be addressed, in the diagnosis, pathogenesis, intervention strategies and therapeutic drugs for AMI with MDD. To aid clinical diagnosis and treatment, this review classifies the existing studies on AMI complicated with MDD, and also includes some of our considerations.
Keywords:
myocardial infarction major depression disorder mechanism intervention strategy myocardial infarction depression1. Introduction
Estimated 17.5 million people die from heart disease per year, of which, acute AMI and its complications account for a large proportion [1]. Clinical statistics show a strong bidirectional association between MDD and AMI [2]. Among people at risk of AMI, the prevalence of MDD is high; about one in five patients have already suffered from MDD at the time of angiography, which indicates that a large proportion of patients had MDD prior to AMI [3,4]. Statistic shows symptoms of MDD doubled the risk for AMI [5].
Studies have found that patients with AMI are three times more likely to suffer from MDD than people without AMI, and patients with MDD after AMI tend to have worse clinical outcomes [6,7]. Better understanding of mechanisms underlying AMI concomitant with MDD will shed light for clinical treatment (Figure 1). This review appraises updated studies of AMI and MDD, and summarizes the current main intervention strategies.
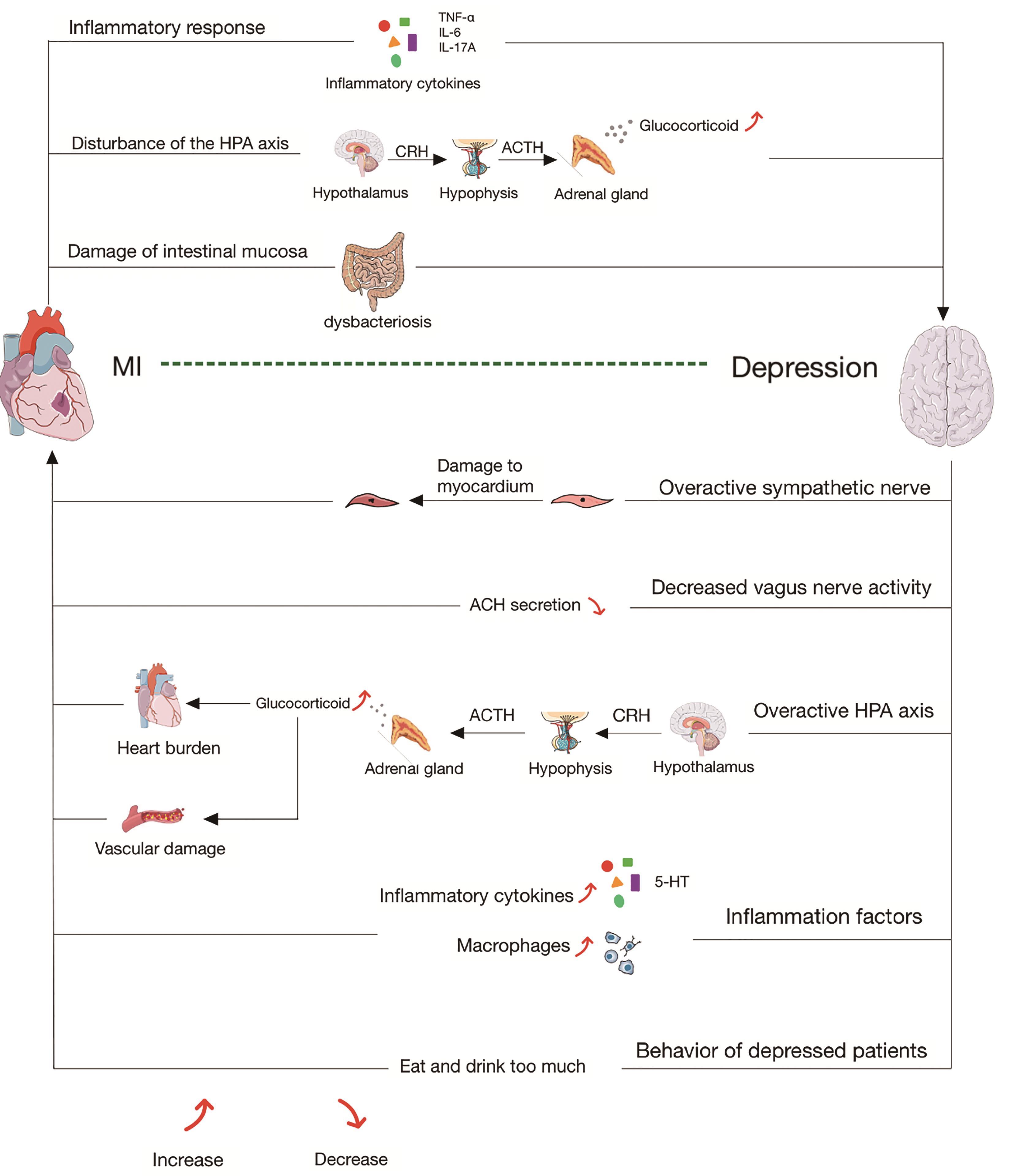
Figure 1. The pathophysiological mechanism of interaction between MDD and AMI. After AMI, (1) Inflammatory cytokines (TNF-α、IL-6、IL-17A) are released and cross the blood-brain barrier, thus leading to MDD. (2) The overactivity of HPA axis lead to massive release of cortisol, and cause MDD. (3) Inflammation factors cause the disorder of intestinal mucosal damage flora, which leads to the disorder of gut-brain axis and result in MDD. Among the people suffering from MDD, (1) Excessive activation of sympathetic nerve causes myocardial cell damage. (2) Reduced vagus nerve activity leads to reduced acetylcholine release, which cause the loss of cardiovascular protection. (3) The overactive HPA axis produces excessive glucocorticoids, resulting in high cardiac load and vascular destruction. (4) A large number of inflammatory factors and macrophage infiltrate, resulting in myocardial destruction. (5) Certain behaviors, such as overeating, during MDD are also risk factors for AMI..
2. Mechanisms of Association between AMI and MDD
2.1. Mechanism of MDD after AMI
2.1.1. Inflammatory Response
Many studies have shown that the inflammatory response is an independent factor leading to MDD after AMI [8,9]. The number of monocytes and neutrophils were increased in the blood of AMI patients. Inflammatory pathways were activated in AMI patients; the plasma concentration of TNF-a, IL-6, IL-17A, IL-12P70 and C-reactive protein were also increased [10-12], which constitute the pathophysiological basis of MDD. After AMI, large amounts of inflammatory cytokines cause destruction of the blood brain barrier (BBB), and induce central nerve inflammation, which leads to MDD [7]. The hypothalamic paraventricular nucleus (PVN) region is the center of autonomic cardiovascular regulation, which is more likely to be affected by BBB leakage after AMI [7]. Interestingly, although myocardial infection and AMI share a similar inflammatory process, myocardial infection does not have a significant impact on MDD [13].
2.1.2. Disturbance of the Hypothalamic-Pituitary-Adrenal (HPA) Axis
HPA axis can enhance sympathetic nerve activity, and MDD is associated with changes in the HPA axis. Hypothalamic neurons can secrete adrenocorticotropin-releasing hormone (CRH), and CRH further promotes the release of adrenocorticotropic hormone (ACTH) in the anterior pituitary, thus promoting the adrenocortical synthesis of serum cortisol and other glucocorticoids [14]. Studies have shown that patients with AMI often also have HPA axis dysfunction, resulting in dysregulation of the cortisol spectrum and hyperfunction of the HPA axis [15,16]. After AMI, due to the stimulation of stress response and various inflammatory mediators, hypothalamus neurons secrete CRH, and the anterior pituitary releases ACTH to promote the synthesis of glucocorticoid by adrenal gland. If this persists, it will lead to the overactivity of the HPA axis and cause MDD.
2.1.3. Damage of Intestinal Mucosa and Imbalance of Intestinal Flora
Growing evidence indicates that the gastrointestinal microbiome is associated with anxiety and depressive disorders. The mechanisms of action may relate to anxiety and MDD pathophysiology through signalling peripheral inflammation to the brain [17]. The microbiome can regulate brain physiology via the gut-microbiota-brain axis [18]. Acute AMI disturbs intestinal flora because of inflammatory factors, psycho-physiological effects and dysfunction of the HPA axis [19]. Alterations in gut microbiome composition could increase the permeability of the gut barrier; activate systemic inflammation and immune responses; regulate the release and efficacy of monoamine neurotransmitters; alter the activity and function of the HPA axis; and modify the abundance of brain-derived neurotrophic factor (BDNF). BDNF plays an important role in MDD through its neurotrophic effect [20]. All these factors eventually lead to MDD [21,22].
2.2. Mechanism of MDD Inducing AMI
2.2.1. Sympathetic Overactivation
The excessive activation of the sympathetic nervous system in patients with MDD is an important mechanism in the progression of cardiovascular disease [23]. Studies have confirmed that depressed patients show increased sympathetic activity, such as increased cardiac sympathicotonus [24]. At the cellular level, chronic sympathetic activation increases oxidative stress and triggers apoptotic pathways. Sustained release or sudden spikes in catecholamine levels also increase risk of cardiac complications, such as arrhythmias and sudden cardiac death, thereby exacerbating myocardial injury [25]. This theory was also proved by the reduced heart rate variability (HRV) in MDD patients. It is well known that a lower HRV is associated with a higher incidence of anxiety, MDD and cardiovascular mortality, while a higher HRV is associated with better cardiovascular function and endurance of stress [26]. This autonomic dysfunction is associated with an increased risk of heart disease in MDD and increased mortality from heart disease [27]. A study by Rakhshan showed that low ischemic tolerance of the heart could be blocked by sympathetic nerve transection, which also suggested that sympathetic overactivation in depressed patients plays an important role in depression-induced AMI [28].
2.2.2. Decreased Vagus Nerve Activity
MDD is associated with reduced vagal nerve activity. Evidence suggests a causal relationship between vagus function and MDD; vagus dysfunction can affect AMI tolerance [29]. On the contrary, vagus nerve stimulation can increase acetylcholine in the cardiac interstitium [30,31]; acetylcholine can dilate myocardial coronary vessels and alleviate injury caused by myocardial ischemia [32,33].
Muscarinic (M2, M3) receptors may contribute to the protective effects of vagal activity and acetylcholine. Antagonism or knockdown of M2, M3 receptors confirms their involvement in the protection of cardiomyocytes [34, 35]. Vagus nerve stimulation (VNS) can also reduce neutrophil invasion and decrease inflammatory marker secretion in the myocardium after ischemia [36]. VNS has been identified as a potential therapy for cardiovascular disorders, including cardiac arrest and acute myocardial infarction. A recent study showed that non-invasive electrical stimulation of the vagus nerve through the skin offers a simple alternative to the established method of vagus nerve stimulation [37]. Despite considerable evidence illustrating the correlation between MDD and reduced vagal nerve activity, and the mechanism of cardiac protection via vagal activity, no study has yet demonstrated that reduced vagal activity aggravates AMI in models of MDD.
2.2.3. Overactivation of the HPA Axis
Hyperactivity of the HPA axis can lead to psychiatric disorders, such as MDD and anxiety disorders [38]. The HPA axis tends to be more active in depressed patients, leading to oversecretion of glucocorticoids and adrenal catecholamines [39]. Glucocorticoids can act directly on the cardiovascular system, causing positive inotropic effects, high blood pressure, and high cardiac output [40,41]. In addition, levels of inflammatory factors in the serum of depressed patients were much higher than that of normal subjects; these inflammatory factors include IL-6, eicosane, platelet activating factor and serotonin [42].
Studies also found that MDD can induce insulin resistance, further promoting hyperglycemia. It is well known that both hyperglycemia and insulin resistance reduce infarct tolerance and aggravate injury [43].
2.2.4. Inflammation Factors
MDD can cause inflammatory cell infiltration, activation of inflammatory signals, and upregulation of TLR4 and NF-κB in chronic stress, MDD models, all these factors exacerbate myocardial injury [44,45]. Evidence showed that chronic stress-induced MDD can also increase macrophage infiltration; this would increase the growth and vulnerability of atherosclerotic plaques, which can easily lead to thrombosis, thus accelerating the occurrence of infarction [46]. As an important inflammatory mediator, 5-HT plays a dual role in promoting platelet aggregation and arterial vasoconstriction; it is considered to be involved in the process of AMI and MDD in many studies. Phosphatidylcreatine-phospholipase (PI-PLC) has the potential to mediate platelet activation through 5-hydroxytryptamine (5-HT) and 5-HT2AR [47], which leads to blood agglutination and is prone to induce AMI [48]. There has also been controversy with the administration of antidepressants because antidepressants can raise serum concentration of 5-HT. Depressed patients may be more prone to thrombosis, which may lead to heart attacks [49].
2.2.5. Behavior of Depressed Patients
Certain behaviors of patients with MDD also have an important impact on heart disease. Low mood in MDD patients may lead to overeating and lack of exercise [50]. Psychological stress, overeating and lack of exercise will result in obesity and diabetes, which will lead to cardiovascular events.
3. Medicine and Intervention Measures for AMI with MDD
Because of the strong association between AMI and MDD, the use of medicine is necessary for patients with MDD or AMI. For patients with AMI, appropriate medications can reduce inflammation and depressive symptoms after MI, resulting in a better prognosis. For patients with MDD, timely application of appropriate drugs can also greatly reduce the possibility of subsequent AMI.
Tricyclic antidepressants (TCAs) and monoamine-oxidase inhibitors (MAOIs) have been discouraged because of their cardiotoxic side effects [31,51]. Drugs that both improve cardiovascular disease and MDD are relatively rare. With deepening understanding of the interactions and mechanism between MDD and AMI, more candidate drugs are assessed for their efficacy in both AMI and MDD. We classified current studies on available marketed drugs in Table 1.
Table 1. Pharmacodynamics study of marketed drugs on AMI and/or MDD.

3.1. Available Marketed Drugs
Among marketed drugs, pentotheobroline (PTX) was found to prevent the increase of pro-inflammatory cytokines in the paraventricular nucleus (PVN) and prefrontal cortex (PFC) in the female rat plasma. It also limits the decrease of brain-derived neurotrophic factor (BDNF), thus alleviates AMI induced depression-like behavior [52]. Minocycline, an antibiotic that inhibits microglial activation and inhibits pro-inflammatory cytokines, can alleviate systemic inflammation, heart failure, and depressive-like behavior after AMI in rats [53]. Trimetazidine is a cardiometabolic drug that regulates 5-HT, 5-HT2AR and SERT in serum and platelets of rats, and has certain antidepressant effects after AMI [54]. Both xinxin pill and sertraline can improve the level of 5-HT2AR and SERT in serum and platelet in rats with AMI and MDD [55].
Apart from animal experiments, statistics of clinical data showed that the use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) can reduce MDD and anxiety scores [56]. Patients taking sertraline can reduce adverse cardiovascular events, but the long-term effects were not significant [57]. Ginkgolides reduce the level of inflammatory cytokines through the STAT-3 pathway, which has great advantages in the treatment of MDD after AMI, and may have a potential effect on patients with post-MI MDD [58]. In a small, randomized, double-blind placebo-controlled trial, the antidepressant fluoxetine was shown to be effective in the treatment of major MDD for three months after AMI [59]. The mechanism of fluoxetine action may be antioxidant and anti-inflammatory [60]. Another randomized, double-blind, placebo-controlled study in patients with AMI found that paroxetine improves cardiac function after AMI by inhibiting G protein-coupled receptor kinase 2 (GRK2), and it is also a widely used antidepressant [61]. Excessive β-adrenergic receptor activity promotes apoptosis and neoplasia; β-blockers have also become the main treatment for patients with coronary heart disease/AMI [62-64]. Zuranolone (Sage-217 /BIIB125) is an active neurosteroid GABAA receptor positive alteration modulator. A randomized, double-blind, placebo-controlled phase III trial found that Zuranolone had better efficacy and safety compared with placebo in adult women with severe postpartum MDD (PPD) [64].
3.2. Developing Medicines and Strategies.
Kai-xin-san reduced depression-induced matrix metalloproteinases (MMPs) expression at mRNA and protein levels; the inhibition of MMPs signaling pathway plays an antidepressant and cardiac protective role [65]. In addition, ginsenosides (GFS) have been shown to inhibit 5-HT transporters, thereby inhibiting 5-HT reuptake and improving post-MI depressive behavior [66]. TNF inhibitors were found to improve depressive symptoms and reduce the risk of cardiovascular events [7]. Two probiotics, Helveticus R0052 and Bifidobacterium R0175, improved symptoms of post-MI MDD by repairing the damaged intestinal barrier and reducing pro-inflammatory factor production [67]. The treatment of human umbilical cord mesenchymal stem cells (HUC-MSCs) can significantly improve the cardiac function of depressed mice after AMI. It reduced myocardial fibrosis and depressive behavior [68].
3.3. Daily Behavior Intervention
Numerous studies have shown that exercise can reduce MDD after AMI, and the effect is more significant in women [5]. Smoking cessation reduces the risk of MDD after AMI [69]. As a practical and non-invasive psychological therapy, eye movement desensitization therapy (EDMR) can effectively improve the symptoms of MDD in patients with AMI [70].
4. Conclusion and Perspectives
Post-MI MDD is also often difficult to detect; among patients who have MDD after AMI, only 25% or less are finally diagnosed with MDD. This is because doctors and patients tend to interpret post-MI MDD as a transient and natural response to life-threatening events, and therefore underestimate the condition, resulting in poor prognosis of patients with post-MI MDD [7].
In conclusion, current literature has demonstrated the involvement of inflammation; HPA axis dysfunction; sympathetic and vagus nerve disorder; imbalance of intestinal flora; patient behavior; and drug side effects. However, the mechanisms behind the correlation between AMI and MDD are complicated and still far from being articulated. Further studies should consider both pathological and psychological factors. This will involve systematic investigation of the heart, peripheral nerves and central nerves. Treatment of AMI complicated with MDD requires more attention from the scientific community. Additional RCTs are needed, including evaluation of non-pharmacologic therapies, such as exercise, to examine the effects of treatment of AMI with MDD on medical and psycho-social outcomes. The current prevailing treatment approach is through drugs and lifestyle changes. For patients with MDD after AMI, it is necessary to carry out timely and proper treatment, which can greatly improve patient prognosis. For patients with MDD, early prevention of AMI is crucial.
Author Contributions: Mingyang Xu and Yinxiang Wei wrote the manuscript. Zhenhui Wang and Yaohui Wang collected literatures. Xiaoming Zhong and Qiying Chen supervised the study and modified the manuscript. All authors reviewed the manuscript.
Funding: National Natural Science Foundation of China (82101913). Clinical Research Project of Shang Municipal Health Commission (202140506)
Data Availability: Not applicable.
Acknowledgments: We thank Professor Jin Bo for revising and midifing the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Sun Z.Q.; Yu T.T.; Ma Y.; et al. Depression and myocardial injury in ST-segment elevation myocardial infarction: A cardiac magnetic resonance imaging study. World. J. Clin. Cases, 2020, 8(7): 1232-1240. DOI: https://doi.org/10.12998/wjcc.v8.i7.1232
- Raič M. Depression and Heart Diseases: Leading Health Problems. Psychiatr. Danub., 2017, 4(Suppl 4): 770-777.
- Carney R.M.; Rich M.W.; Freedland K.E.; et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom. Med., 1988, 50(6): 627-33. DOI: https://doi.org/10.1097/00006842-198811000-00009
- Cocchio S.; Baldovin T.; Furlan P.; et al. Is depression a real risk factor for acute myocardial infarction mortality? A retrospective cohort study. BMC Psychiatry, 2019, 19(1): 122. DOI: https://doi.org/10.1186/s12888-019-2113-8
- Kjellström B.; Gustafsson A.; Nordendal E.; et al. Symptoms of depression and their relation to myocardial infarction and periodontitis. Eur. J. Cardiovasc. Nurs., 2017, 16(6): 468-474. DOI: https://doi.org/10.1177/1474515116686462
- Bot M.; Pouwer F.; Zuidersma M.; et al. Association of coexisting diabetes and depression with mortality after myocardial infarction. Diabetes Care, 2012, 35(3): 503-9. DOI: https://doi.org/10.2337/dc11-1749
- Liu H.; Luiten P.G.; Eisel U.L.; et al. Depression after myocardial infarction: TNF-α-induced alterations of the blood-brain barrier and its putative therapeutic implications. Neurosci. Biobehav. Rev., 2013, 37(4): 561-72. DOI: https://doi.org/10.1016/j.neubiorev.2013.02.004
- Wachowska K.; Bliźniewska-Kowalska K.; Sławek J.; et al. Common pathomechanism of migraine and depression. Psychiatr. Pol., 2022, 17:1-15.
- Beurel E.; Toups M.; Nemeroff C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron, 2020, 107(2): 234-256. DOI: https://doi.org/10.1016/j.neuron.2020.06.002
- Wilkowska A.; Pikuła M.; Rynkiewicz A.; et al. Increased plasma pro-inflammatory cytokine concentrations after myocardial infarction and the presence of depression during next 6-months. Psychiatr. Pol., 2015, 49(3): 455-64. DOI: https://doi.org/10.12740/PP/33179
- Saparov A.; Ogay V.; Nurgozhin T.; et al. Role of the immune system in cardiac tissue damage and repair following myocardial infarction. Inflamm. Res., 2017, 66(9): 739-751. DOI: https://doi.org/10.1007/s00011-017-1060-4
- de Kleijn D.P.V.; Chong S.Y.; Wang X.; et al. Toll-like receptor 7 deficiency promotes survival and reduces adverse left ventricular remodelling after myocardial infarction. Cardiovasc. Res., 2019, 115(12): 1791-1803. DOI: https://doi.org/10.1093/cvr/cvz057
- Jovanova O.; Luik A.I.; Leening M.J.; et al. The long-term risk of recognized and unrecognized myocardial infarction for depression in older men. Psychol. Med., 2016, 46(9): 1951-60. DOI: https://doi.org/10.1017/S0033291716000544
- Sapolsky R.M.; Romero L.M.; Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev., 2000, 21(1): 55-89. DOI: https://doi.org/10.1210/edrv.21.1.0389
- Headrick J.P.; Peart J.N.; Budiono B.P.; et al. The heartbreak of depression: 'Psycho-cardiac' coupling in myocardial infarction. J. Mol. Cell. Cardiol., 2017, 106: 14-28. DOI: https://doi.org/10.1016/j.yjmcc.2017.03.007
- Wu P.; Vaseghi M. The autonomic nervous system and ventricular arrhythmias in myocardial infarction and heart failure. Pacing. Clin. Electrophysiol., 2020, 43(2): 172-180. DOI: https://doi.org/10.1111/pace.13856
- Simpson C.A.; Diaz-Arteche C.; Eliby D.; et al. The gut microbiota in anxiety and depression - A systematic review. Clin. Psychol. Rev., 2021, 83: 101943. DOI: https://doi.org/10.1016/j.cpr.2020.101943
- Cenit M.C.; Sanz Y.; Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World. J. Gastroenterol., 2017, 23(30): 5486-5498. DOI: https://doi.org/10.3748/wjg.v23.i30.5486
- Wu Z.X.; Li S.F.; Chen H.; et al. The changes of gut microbiota after acute myocardial infarction in rats. PLoS. One., 2017, 12(7): e0180717. DOI: https://doi.org/10.1371/journal.pone.0180717
- Gelle T.; Samey R.A.; Plansont B.; et al. BDNF and pro-BDNF in serum and exosomes in major depression: Evolution after antidepressant treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry., 2021, 109: 110229. DOI: https://doi.org/10.1016/j.pnpbp.2020.110229
- Du Y.; Gao X.R.; Peng L.; et al. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon, 2020, 6(6): e04097. DOI: https://doi.org/10.1016/j.heliyon.2020.e04097
- Yang Y.; Li X.; Chen S.; et al. Mechanism and therapeutic strategies of depression after myocardial infarction. Psychopharmacology (Berl)., 2021, 238(6): 1401-1415. DOI: https://doi.org/10.1007/s00213-021-05784-0
- Malpas S.C. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol. Rev., 2010, 90(2): 513-57. DOI: https://doi.org/10.1152/physrev.00007.2009
- Barton D.A.; Dawood T.; Lambert E.A.; et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J. Hypertens., 2007, 25(10): 2117-24. DOI: https://doi.org/10.1097/HJH.0b013e32829baae7
- Tachibana H.; Naga Prasad S.V.; Lefkowitz R.J.; et al. Level of beta-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation, 2005, 111(5): 591-7. DOI: https://doi.org/10.1161/01.CIR.0000142291.70954.DF
- Bair A.; Marksteiner J.; Falch R.; et al. Features of autonomic cardiovascular control during cognition in major depressive disorder. Psychophysiology. 2021, 58(1): e13628. DOI: https://doi.org/10.1111/psyp.13628
- Carney R.M.; Saunders R.D.; Freedland K.E.; et al. Association of depression with reduced heart rate variability in coronary artery disease. Am. J. Cardiol., 1995, 76(8): 562-4. DOI: https://doi.org/10.1016/S0002-9149(99)80155-6
- Rakhshan K.; Imani A.; Faghihi M.; et al. Evaluation of Chronic Physical and Psychological Stress Induction on Cardiac Ischemia / Reperfusion Injuries in Isolated Male Rat Heart: The Role of Sympathetic Nervous System. Acta. Med. Iran., 2015, 53(8): 482-90.
- Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol. Psychol., 2007, 74(2): 200-11. DOI: https://doi.org/10.1016/j.biopsycho.2005.08.010
- Kawada T.; Akiyama T.; Shimizu S.; et al. Detection of endogenous acetylcholine release during brief ischemia in the rabbit ventricle: a possible trigger for ischemic preconditioning. Life Sci., 2009, 85(15-16): 597-601. DOI: https://doi.org/10.1016/j.lfs.2009.08.015
- Post-Myocardial Infarction Depression Clinical Practice Guideline Panel. AAFP guideline for the detection and management of post-myocardial infarction depression. Ann. Fam. Med., 2009, 7(1): 71-9. DOI: https://doi.org/10.1370/afm.918
- Calvillo L.; Vanoli E.; Andreoli E.; et al. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J. Cardiovasc. Pharmacol., 2011, 58(5): 500-7. DOI: https://doi.org/10.1097/FJC.0b013e31822b7204
- ShinlapawittayatornK.; ChindaK.; PaleeS.; et al. Vagus nerve stimulation initiated late during ischemia, but not reperfusion, exerts cardioprotection via amelioration of cardiac mitochondrial dysfunction. Heart Rhythm, 2014, 11(12): 2278-87. DOI: https://doi.org/10.1016/j.hrthm.2014.08.001
- Zhao J.; Su Y.; Zhang Y.; et al. Activation of cardiac muscarinic M3 receptors induces delayed cardioprotection by preserving phosphorylated connexin43 and up-regulating cyclooxygenase-2 expression. Br. J. Pharmacol., 2010, 159(6): 1217-25. DOI: https://doi.org/10.1111/j.1476-5381.2009.00606.x
- Hu H.; Qi L.; Ren C.; et al. M2 Macrophage-Derived Exosomes Regulate Myocardial Ischemia-Reperfusion And Pyroptosis Via ROS/NLRP3 Pathway. Heart Surg. Forum, 2022, 25(5): E698-E708. DOI: https://doi.org/10.1532/hsf.4919
- Yi C.; Zhang C.; HuX.; et al. Vagus nerve stimulation attenuates myocardial ischemia/reperfusion injury by inhibiting the expression of interleukin-17A. Exp. Ther. Med., 2016, 11(1): 171-176. DOI: https://doi.org/10.3892/etm.2015.2880
- Murray A.R.; Atkinson L.; MahadiM.K., et al. The strange case of the ear and the heart: The auricular vagus nerve and its influence on cardiac control. Auton. Neurosci., 2016, 199: 48-53. DOI: https://doi.org/10.1016/j.autneu.2016.06.004
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects. Compr. Physiol., 2014, 4(2): 715-38. DOI: https://doi.org/10.1002/cphy.c130036
- Eskandari F.; Sternberg E.M. Neural-immune interactions in health and disease. Ann. N. Y. Acad. Sci., 2002, 966: 20-7. DOI: https://doi.org/10.1111/j.1749-6632.2002.tb04198.x
- Goodwin J.E. Glucocorticoids and the Cardiovascular System. Adv. Exp. Med. Biol., 2015, 872: 299-314. DOI: https://doi.org/10.1007/978-1-4939-2895-8_13
- MacLeod C.; Hadoke P.W.F.; Nixon M. Glucocorticoids: Fuelling the Fire of Atherosclerosis or Therapeutic Extinguishers? Int. J. Mol. Sci., 2021, 22(14): 7622. DOI: https://doi.org/10.3390/ijms22147622
- Tsigos C.; Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res., 2002, 53(4): 865-71. DOI: https://doi.org/10.1016/S0022-3999(02)00429-4
- Angeli F.; Reboldi G.; Poltronieri C.; et al. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther. Adv. Cardiovasc. Dis., 2015, 9(6): 412-24. DOI: https://doi.org/10.1177/1753944715594528
- Wang R.P.; Yao Q.; Xiao Y.B.; et al. Toll-like receptor 4/nuclear factor-kappa B pathway is involved in myocardial injury in a rat chronic stress model. Stress, 2011, 14(5): 567-75. DOI: https://doi.org/10.3109/10253890.2011.571729
- Matsuura N.; Nagasawa K.; Minagawa Y.; et al. Restraint stress exacerbates cardiac and adipose tissue pathology via β-adrenergic signaling in rats with metabolic syndrome. Am. J. Physiol. Heart. Circ. Physiol., 2015, 308(10): H1275-86. DOI: https://doi.org/10.1152/ajpheart.00906.2014
- Roth L.; Rombouts M.; Schrijvers D.M.; et al. Chronic intermittent mental stress promotes atherosclerotic plaque vulnerability, myocardial infarction and sudden death in mice. Atherosclerosis, 2015, 242(1): 288-94. DOI: https://doi.org/10.1016/j.atherosclerosis.2015.07.025
- Guo L.; Hu S. PI-PLC signal pathway: a possible pathogenesis link post-myocardial infarction to depression. Med. Hypotheses, 2009, 73(2): 156-7. DOI: https://doi.org/10.1016/j.mehy.2009.02.032
- Liu M.Y.; Ren Y.P.; Wei W.L.; et al. Changes of Serotonin (5-HT), 5-HT2A Receptor, and 5-HT Transporter in the Sprague-Dawley Rats of Depression, Myocardial Infarction and Myocardial Infarction Co-exist with Depression. Chin. Med. J., 2015, 128(14): 1905-9. DOI: https://doi.org/10.4103/0366-6999.160526
- Parkin L.; Balkwill A.; Green J.; et al. Depression, anxiety, psychotropic drugs, and acute myocardial infarction: large prospective study of United Kingdom women. Psychol. Med., 2023, 53(4): 1576-1582. DOI: https://doi.org/10.1017/S0033291721003159
- Zhang L.J.; Liu M.Y.; Rastogi R.; et al. Psychocardiological disorder and brain serotonin after comorbid myocardial infarction and depression: an experimental study. Neurol. Res., 2018, 40(6): 516-523. DOI: https://doi.org/10.1080/01616412.2018.1455460
- Jiang W.; Davidson J.R. Antidepressant therapy in patients with ischemic heart disease. Am, Heart. J., 2005, 150(5): 871-81. DOI: https://doi.org/10.1016/j.ahj.2005.01.041
- Najjar F.; Ahmad M.; Lagace D.; et al. Role of Myocardial Infarction-Induced Neuroinflammation for Depression-Like Behavior and Heart Failure in Ovariectomized Female Rats. Neuroscience, 2019, 415: 201-214. DOI: https://doi.org/10.1016/j.neuroscience.2019.07.017
- Wang H.W.; Ahmad M.; Jadayel R.; et al. Inhibition of inflammation by minocycline improves heart failure and depression-like behaviour in rats after myocardial infarction. PLoS One, 2019, 14(6): e0217437. DOI: https://doi.org/10.1371/journal.pone.0217437
- Zhang Y.; Chen Y.; Ma L. Depression and cardiovascular disease in elderly: Current understanding. J. Clin. Neurosci., 2018, 47: 1-5. DOI: https://doi.org/10.1016/j.jocn.2017.09.022
- Liu M.Y.; Zhang L.J.; Zhou Y.X.; et al. 5-Hydroxytryptamine Changes under Different Pretreatments on Rat Models of Myocardial Infarction and/or Depression. Chin. Med. J., 2017, 130(18): 2219-2225. DOI: https://doi.org/10.4103/0366-6999.213966
- Sarkar S.; Chadda R.K.; Kumar N.; et al. Anxiety and depression in patients with myocardial infarction: findings from a centre in India. Gen. Hosp. Psychiatry, 2012, 34(2): 160-6. DOI: https://doi.org/10.1016/j.genhosppsych.2011.09.016
- Serebruany V.L.; Glassman A.H.; Malinin A.I.; et al. Sertraline AntiDepressant Heart Attack Randomized Trial Study Group. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation, 2003, 108(8): 939-44. DOI: https://doi.org/10.1161/01.CIR.0000085163.21752.0A
- Ge Y.; Xu W.; Zhang L.; et al. Ginkgolide B attenuates myocardial infarction-induced depression-like behaviors via repressing IL-1β in central nervous system. Int. Immunopharmacol., 2020, 85: 106652. DOI: https://doi.org/10.1016/j.intimp.2020.106652
- Strik J.J.; Honig A.; Lousberg R.; et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom. Med., 2000, 62(6): 783-9. DOI: https://doi.org/10.1097/00006842-200011000-00007
- Yaman O.M.; ErmanH.; GunerI.; et al. Remote myocardial injury: the protective role of fluoxetine. Can. J. Physiol. Pharmacol. 2018, 96(4): 319-327. DOI: https://doi.org/10.1139/cjpp-2017-0383
- Schumacher S.M.; Gao E.; Zhu W.; et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci. Transl. Med., 2015, 7(277): 277ra31. DOI: https://doi.org/10.1126/scitranslmed.aaa0154
- Remondino A.; Kwon S.H.; CommunalC.; et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ. Res., 2003, 92(2): 136-8. DOI: https://doi.org/10.1161/01.RES.0000054624.03539.B4
- Yu Q.J.; Si R.; Zhou N.; et al. Insulin inhibits beta-adrenergic action in ischemic/reperfused heart: a novel mechanism of insulin in cardioprotection. Apoptosis, 2008, 13(2): 305-17. DOI: https://doi.org/10.1007/s10495-007-0169-2
- Shin S.Y.; Kim T.; Lee H.S.; et al. The switching role of β-adrenergic receptor signalling in cell survival or death decision of cardiomyocytes. Nat. Commun., 2014, 5: 5777. DOI: https://doi.org/10.1038/ncomms6777
- Hu Y.; Dong X.; Zhang T.; et al. Kai Xin San suppresses matrix metalloproteinases and myocardial apoptosis in rats with myocardial infarction and depression. Mol. Med. Rep., 2020, 21(1): 508-516. DOI: https://doi.org/10.3892/mmr.2019.10807
- Liu M.Y.; Ren Y.P.; Zhang L.J.; et al. Pretreatment with Ginseng Fruit Saponins Affects Serotonin Expression in an Experimental Comorbidity Model of Myocardial Infarction and Depression. Aging. Dis., 2016, 7(6): 680-686. DOI: https://doi.org/10.14336/AD.2016.0729
- Arseneault-Bréard J.; Rondeau I.; Gilbert K.; et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr., 2012, 107(12): 1793-9. DOI: https://doi.org/10.1017/S0007114511005137
- Zhang Y.; Wang X.; Li Y.; et al. Human umbilical cord mesenchymal stem cells ameliorate depression by regulating Jmjd3 and microglia polarization in myocardial infarction mice. Psychopharmacology, 2021, 238(10): 2973-2984. DOI: https://doi.org/10.1007/s00213-021-05912-w
- Bernard P.; Ninot G.; Moullec G.; et al. Smoking cessation, depression, and exercise: empirical evidence, clinical needs, and mechanisms. Nicotine. Tob. Res., 2013, 15(10): 1635-50. DOI: https://doi.org/10.1093/ntr/ntt042
- Behnammoghadam M.; Alamdari A.K.; Behnammoghadam A.; et al. Effect of Eye Movement Desensitization and Reprocessing (EMDR) on Depression in Patients With Myocardial Infarction (MI). Glob. J. Health Sci., 2015; 7(6): 258-62. DOI: https://doi.org/10.5539/gjhs.v7n6p258






