
Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Mini Review
Injectable Multifunctional Natural Polymer-Based Hydrogels for the Local Delivery of Therapeutic Agents
Xue Bai 1,Annalisa Tirella1,2,*
1 Division of Pharmacy and Optometry, School of Health Science, Faculty of Biology, Medicine and Health, University of Manchester, Oxford Road, Manchester M13 9PT, UK.
2 BIOtech-Center for Biomedical Technologies, Department of Industrial Engineering, University of Trento, Via delle Regole 101, Trento 38123, Italy.
* Correspondence: annalisa.tirella@unitn.it , annalisa.tirella@manchester.ac.uk (Annalisa Tirella).
Received: 13 November 2022
Accepted: 15 December 2022
Published: 21 December 2022
Abstract: Hydrogels are water-based polymeric three-dimensional network with advantageous properties for the delivery of bioactive components, ranging from small therapeutic agents to therapeutic cells. Natural-based hydrogels have great potential as delivery vehicles for the local controlled release of therapeutic agents at the target site. Injectable hydrogels are designed to load therapeutic agents by simple mixing within the polymer solutions, as well as use nanoparticles able to respond to specific external conditions, such as temperature and pH. Herein, we present an overview of the properties of natural injectable hydrogels and recent developments for their use to control the local release of therapeutic agents; as well as strategies to crosslink in-situ multifunctional injectable hydrogels that act as therapeutical depot system. The mini review focuses on alginate-based injectable hydrogels as controlled drug delivery systems, presenting advantages and challenges of their application in cancer therapy
Keywords:
alginate injectable hydrogels local drug delivery natural polymers1.Introduction
Among the active compounds developed as therapeutic agents, a recurrent issue for their use clinically resides on the poor control over the body distribution and the limited dose at the site of action, hence their efficacy. Different strategies, such as route of administration and dosing, have been used to better control the delivery of newly developed therapeutic agents; however, the dose at the target tissue and the plasma concentration remain often below the required efficacy levels. As an example, a typical strategy used to increase the concentration of a therapeutic agent at the site of action is to increase the frequency of administration; leading to known effects such as reduced patient compliance and increases possibilities of overdose [1].
To overcome these issues, newly developed therapeutic agents have been formulated as controlled drug delivery systems (DDS) and using alternative routes of administration. In this case, DDS are designed to release single or multiple therapeutic agents at a constant and known rate and at a known site (control in time and space), with the ultimate scope to maintain a constant concentration of the therapeutic agent in the blood and at the site of action. Reported DDS are formulated as tumour-targeting nanoparticles, microsphere-depots, transdermal patches, functionalized implants and antibody-drug conjugates [2]. Main drawbacks of such DDS are poor material biocompatibility, possible systemic toxicity, surgical removal when too large, and high associated manufacturing costs. A new strategy, investigated over the past decade, foresees the inclusion of the therapeutic agents within a polymeric hydrogel network to be easily injected at the target site and that uses different release mechanisms (e.g. diffusion, swelling) to control their release [3].
Injectable hydrogels loaded with therapeutic agents (Figure 1) is a rather new class of DDS, used to overcome some of the above-mentioned issues with conventional DDS. Hydrogels are water-swollen and cross-linked networks, typically composed of synthetic or natural polymers, and their blends [4]. Natural polymers, such as alginate, collagen, chitosan and hyaluronic acid (HA) form hydrogels with properties similar to the ones of the extracellular matrix (ECM), as well as being inherently biodegradable and often having integrin binding motifs allowing cell adhesion [5]. Synthetic polymers can also mimic certain properties of ECM, as well as include other active motifs to better control the release of therapeutic agents in space and time. Both natural and synthetic polymers can be selectively modified and formulated in hydrogel-precursor low viscous solution, which can be easily injected with standard needles (ideal needle gauge ranges between 22 and 25) and crosslinked at the site of action (in situ) using several strategies to form covalent or reversible crosslinks with non-toxic crosslinking reactions, or by-product [6,7]. Therapeutic agents and traditional controlled DDS can be mixed at a known concentration in hydrogel precursors, injected with minimally invasive techniques and form hydrogel-depot adhering to the surrounding tissues, allowing precise control over the loading, diffusion and release of the therapeutic agents [8].
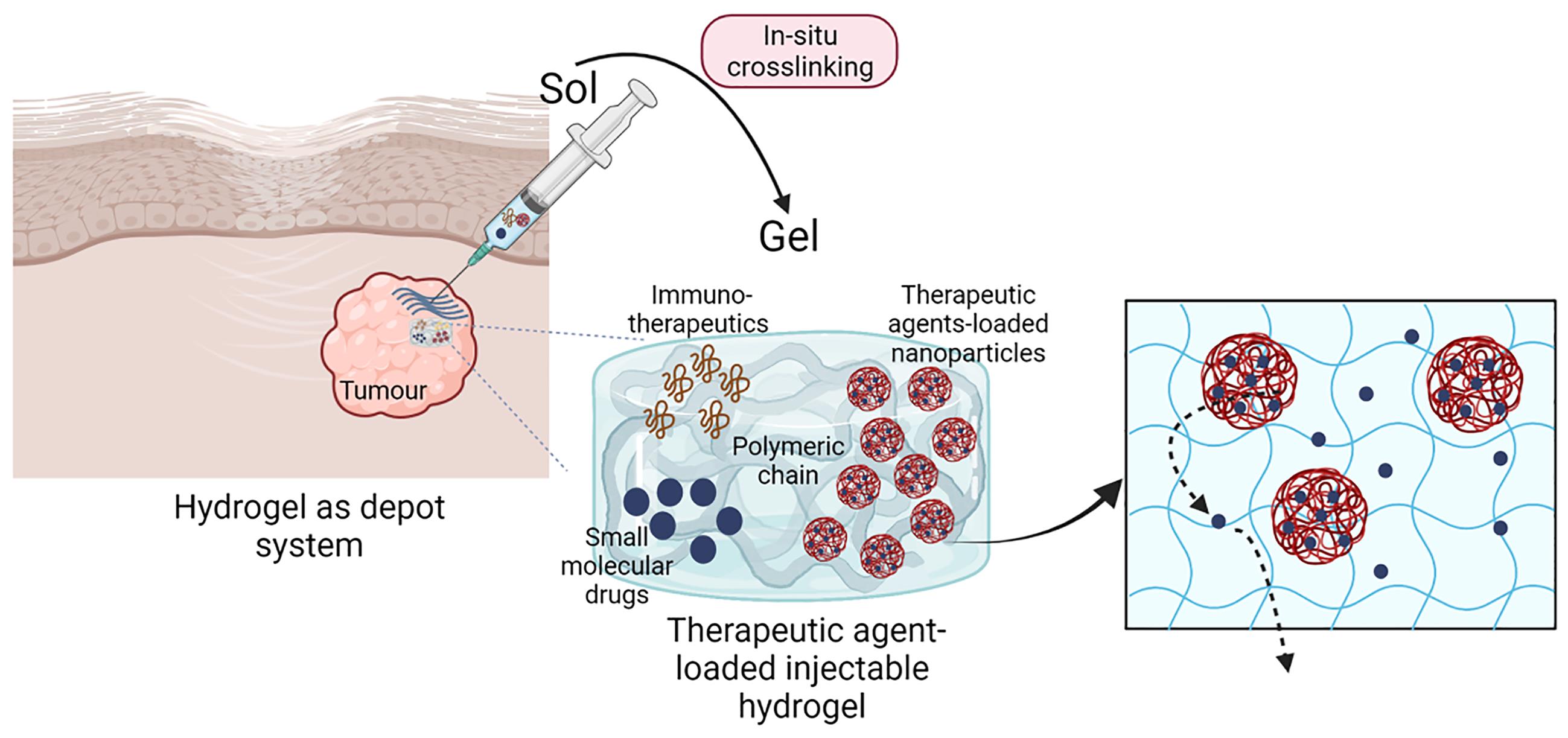
Figure 1. Schematic illustration of injectable hydrogel as drug delivery systems. Composite and/or multifunctional hydrogels are easily injected and gelled in-situ (e.g. intra-tumoral), crosslinks allow the formation of a stable hydrogel that containing a known dose of therapeutic agents. As sketched, these could be soluble free drug, drug-loaded nanoparticles and/or immunotherapeutic agents. Injected hydrogels enable the precise dosing in space (depot system) and time (controlled drug release) of therapeutic agents, and represents promising alternative to conventional therapies used for the treatment of solid tumours (Created with BioRender.com).
The increased number of publications over the past 5-year on injectable hydrogels as DDS reflects how the next generation of biomaterials and new formulation strategies for the effective delivery of therapeutics (Figure 2), and the advancements required to improve bioavailability of therapeutic agents, hence efficacy [9]. Scope of this mini review is to summarize the key properties of natural polymers used as injectable hydrogels for the controlled drug release, and in particular to highlight advantages and limitations on the use of composite alginate hydrogels for the intra-tumoral (IT) delivery of therapeutic agents.
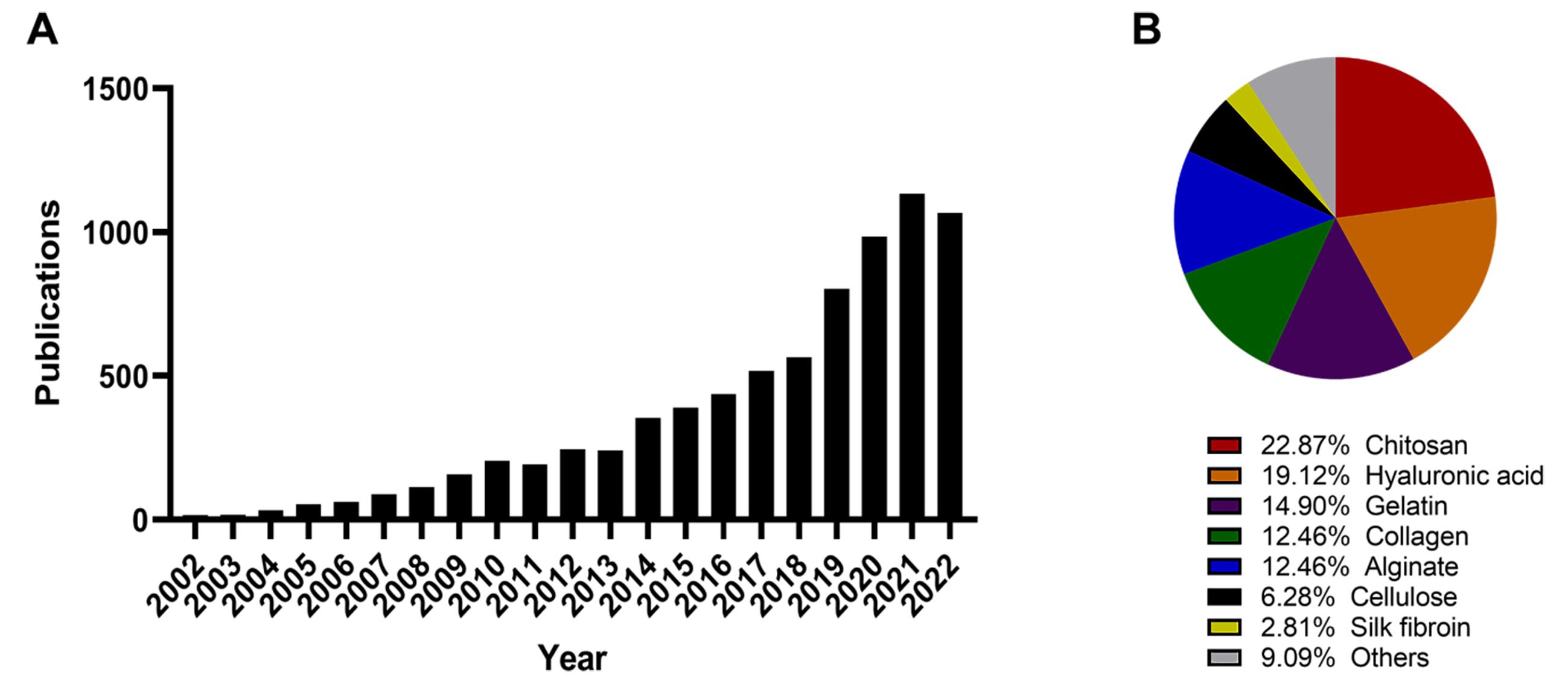
Figure 2. Use of injectable hydrogels in the past 20-year: a Web of Science search with the terms “search in: all database, “all collections”/“topic: injectable hydrogels”/“Year: 2002‒2022” conducted on 04/11/2002. The number of publications published on the topic “injectable hydrogel” since 2002 returned a total of 7767 results. Results grouped per year of publication, showing an increase in publication over the past 5-year (A). Refinement of research within the primary search on the biomaterials used: results mentioning in the title and/or abstract the on the type of biomaterial used to prepare injectable hydrogel was assessed: “Alginate”, “Chitosan”, “Collagen”, “Gelatin”, “Hyaluronic acid”, “Cellulose”, and “Silk fibroin” (B). The pie chart shows the proportion of articles mentioning the type of biomaterial, and each category is colour coded as follows: Alginate (blue), Chitosan (red), Collagen (green), Gelatin (violet), Hyaluronic acid (orange), Cellulose (black), Silk fibroin (yellow) and Others (grey).
2.Injectable Hydrogels Using Natural Biomaterials
Hydrogels are generally classified as natural and synthetic, based on the materials used in their formulation. Natural polymer-based hydrogels offer more advantages to the synthetic counterpart, being biodegradable, biocompatible, sustainable and low cost (e.g. derived from food industry by-products), and typically they limit inflammatory processes and/or immune responses in host tissues [10,11]. The more common natural polymers used to formulate injectable hydrogels in healthcare and pharmaceutical application are summarized in Table 1.
Table 1 Natural biomaterials used for the formulation of injectable hydrogels and pharmaceutical applications, summarizing the main advantages and disadvantages for their use as DDS.

Another classification of hydrogels relies on the type of crosslinks formed within the network: covalent crosslinks (non-reversible) form a stable hydrogel network, whereas hydrogels with physical crosslinks have the possibility to change their state thanks to the reversible nature of crosslinks. Important characteristics of injectable hydrogels is their stability in physiological conditions upon injection and prolonged until degradation. Hydrogel stability can be controlled tuning the type of crosslinks, the composition of the hydrogel and the functionality of polymers (e.g. enzymatic-degradable motifs). Examples of covalently crosslinked hydrogels are glutaraldehyde cross-linked chitosan and photo-crosslinked methacrylated gelatin [21].
3.Hydrogels for Intra-Tumoral Cancer Therapy
3.1. Advantages of Intra-Tumoral Cancer Therapy
As discussed above, the repeated administration of therapeutic agents (such as anticancer drugs) does not guarantee the required therapeutic agent concentration at the tumour site. For example, in the case of solid tumours, IT delivery is used as alternative route of administration to increase the concentration within the tumour mass and enhance efficacy of the treatment [22]. Many are the advantages of IT delivery, improving efficacy and reducing patient morbidity via: (1) controlled drug distribution in space and time, ensuring adequate targeting to cancer cells; (2) dose reduction and reduced dose frequency, ensuring required drug concentration levels at the tumour site as IT delivery bypass the bloodstream; and (3) reduced off-target delivery, thereby minimizing systemic toxicity [22,23].
The main disadvantage in using IT delivery occurs when the delivered formulation is liquid or has low viscosity. In this case, the loaded therapeutic agent can diffuse quickly out of the injected site, causing similar adverse effects to IV delivery. Increasing the viscosity of injections may not be advantageous, with more associated risks due to poor syringeability and discomfort to the patient. In this context, in-situ forming hydrogels with low viscosity precursor solution represent a valid alternative, and offer a great advantage in terms of local delivery and efficacy. The use of multifunctional polymers enables ease injectability, rapid gelation (e.g. photo-crosslink) able to retain therapeutic agents and control their release in time and space in the tumour mass [22].
3.2. Natural Hydrogels as Depot Systems for Intra-Tumoral Delivery: In-Situ Crosslinking Methods
Injectable natural polymers forming hydrogel in-situ are shown to be less invasive and more effective in the treatment of many confined pathologies, such as solid tumours. Several gelation strategies can be used to form covalent and physical crosslinks and obtain hydrogels with controlled physico-chemical properties and prolonged stability in vivo, such as photo-polymerization, chemical, enzymatic, and physical crosslinking (e.g. pH-sensitive, thermo-sensitive) [22]. Additionally, and to control the release of therapeutic agents, it is important to well-design into hydrogels polymers crosslinking type and density, therapeutic agent/polymer affinity, and/or polymer degradation rates. Of note, therapeutic agents can be covalently linked to the polymer with their release controlled by hydrolysable bonds or exposure to external triggers (e.g. UV-vis light, ultrasound, magnetic fields) [24].
Photo-polymerization. Photo-sensitive motifs can be linked to polymers, enabling precisely crosslinking formation in space and time upon exposure to a known light source (wavelength, energy) [22]. Methacrylation of natural polymers is a common strategy used to add photo-sensitive motifs to polymers [11]; and recently being used in combination with gelation strategies (e.g. thermal gelation) [25] to better control the release of therapeutic agents. Obara et al. reported on the use of a photo-sensitive chitosan-derived formulation (UV-sensitive by azide and lactose groups) to obtain paclitaxel-loaded insoluble hydrogels [26]. The system release 35–40% of the paclitaxel within 1 day, followed by a gradual release within 3 days in-vitro; showing that photo-crosslinked chitosan hydrogels are stable in-vivo for more than 1-month and that paclitaxel-incorporated hydrogel prevented tumour expansion more effectively than the free paclitaxel in-vivo [6].
Chemical crosslinking. Natural polymers possess many terminal groups such as primary amines and carboxylic acid functional groups, or can be functionalized with additional specific functional groups, which can be used to control the gelation and the resultant hydrogels’ properties [22]. Emoto et al. reported on the use of a modified HA (HA-oxidized and HA-adipic dihydrazide) to formulate injectable systems for the sustained release of cisplatin. Obtained HA-based hydrogels are able to crosslink rapidly in-vivo (20‒100 s) without forming any toxic by-product and release cisplatin within 4 days, reducing the weight of peritoneal nodules [27].
Physical crosslinking. Many natural polymers are able to form physical hydrogels by changing the temperature, pH, ions concentration, or by mixing polyanion and polycation solutions [28,29]. Gelatin is possibly the most used thermo-sensitive natural polymer in biomedical applications, with the main drawback of being unstable at physiological temperatures. Poly(N-isopropyl acrylamide) (PNIPAAM) is instead the most used synthetic thermos-sensitive polymer, able to switch from liquid to solid network at physiological temperature; thanks to its property, natural-based polymers are grafted to PNIPAAM, but reported to have lower stability in-vivo compare to the synthetic hydrogel. Chitosan-derived thermo-sensitive hydrogels have been used over the past decades, with examples of paclitaxel loaded hydrogels designed for IT delivery. In a study from Ruel-Gariépy et al., chitosan neutralized with β-glycerophosphate was formulated as liquid precursor at room temperature (easy to inject), being able to form stable hydrogels at physiological temperatures able to release loaded paclitaxel up to 1-month, showing reduced tumour mass growth in-vivo [30].
pH-mediated crosslinking is also widely used, mainly for the treatment of skin conditions and wound healing applications. Functionalization of polymers is required to enable precise control over interactions between therapeutic and polymers in pH range of 5.5–8.5. Blends of natural and synthetic polymers, for example N-carboxyethyl chitosan and dibenzaldehyde-terminated poly(ethylene glycol) (PEGDA) precursor, were reported to be injected in-situ via 22G needle and form hydrogels loading doxorubicin with a controlled release (over a week) at rates proportional to the pH value (lower pH promote higher degradation, hence faster release) [31]. In another study by Zhao et al., glycol chitosan/benzaldehyde capped with poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) were formulated as pH-sensitive amphiphilic hydrogels for the co-delivery of doxorubicin (hydrophilic) and paclitaxel (hydrophobic). Hydrogels formed rapidly (50-500 s), loaded the required dose of both drugs, and showed a pH-dependent release: in-vivo studies showed that hydrogels with high doxorubicin and moderate paclitaxel dose were well tolerated and able to inhibit tumour growth [32].
3.3. Alginate-Based Injectable Systems for Local and Controlled Drug Release: Intra-Tumoral Cancer Therapy
Alginates are linear polysaccharides widely used in biomedical applications, such as regenerative medicine, wound healing, and drug delivery. When used in combination with other biomaterials, for example nanoparticles or solid drugs, alginate-based hydrogels offer peculiar properties, advantageous for the controlled delivery of therapeutic agents to solid tumours, as recently reviewed by Iravani and Varma [33]. Many studies used alginate-based injectable systems for the controlled delivery of different therapeutic agents (Table 1, Figure 2). Zhao et al. used methacrylated alginate-based hydrogel for the controlled delivery of water-soluble macromolecules and protein, with prolonged (over 2-weeks) localized release at physiological pH [25]. Chao et al. developed alginate-based systems for localised chemoimmunotherapy. Unmodified alginate mixed with chemotherapeutic drug and an immune adjuvant rapidly formed physical hydrogels upon IT injection due to the presence of calcium ions (Ca2+) within the tumour. After the in-situ gelation, alginate-based hydrogels were reported to slowly released the therapeutic agents to the tumour mass, with proven tumour growth inhibition and immune memory effect in several orthotopic tumour models (e.g. breast, brain) [34]. Ferreira et al. reported on alginate-based injectable hydrogels to reduce angiogenesis in tumours delivering the monoclonal antibody bevacizumab [35]. Alamzadeh et al. used alginate hydrogels co-loaded with cisplatin and gold nanoparticles as multifunctional system to combine photothermal therapy, chemotherapy, and radiation therapy [36]. Xiao et al. formulated even more sophisticated smart alginate-based multifunctional hydrogels: alginate with glucose oxidase-conjugated polyacrylic acid-stabilized iron oxide nanoparticles and Toll-like receptor 7/8 agonist resiquimod (R848) was injected and hydrogels formed by ionic cross-linking with physiological Ca2+ level within the tumour mass. This system promoted starvation strategy (catalyzed conversion of glucose into gluconic acid and hydrogen peroxide), chemodynamic therapy (as result of the first conversion) and tumor-associated macrophages repolarization (increased M1/M2 ratio), with observed tumour growth inhibition in-vivo [37]. All these (and many other studies) claim that alginate-based injectable systems have the potential to lower the administered dosage of each individual therapy, reducing adverse side effects and increased patient compliance to therapy.
4.Conclusions
The past decades have seen increased research on the use of natural polymers and their formulation as injectable systems for controlled drug release (Figure 2). Within these, alginate is the natural polymer with peculiar properties, such as rapid gelation (ionic cross-linking with physiological Ca2+ lions) and stability in physiological condition (non-degradable by mammalian enzymes), and tuneable crosslink type and density (inclusion of functional motifs on alginate chain). Alginate-based injectable systems can deliver different therapeutic agents within solid tumours, reducing systemic side-effect often reported in traditional drug delivery systems.
There are currently more than 30 injectable hydrogel products approved by the FDA and/or EMA for the treatment of various diseases, and a greater number is currently investigated pre-clinically [38]. Although physically crosslinked injectable hydrogels (such as alginate) have some known limitations (e.g. stability, rapid release of hydrophilic drugs) not evidenced in chemical crosslinked hydrogels, the latter have often toxic by-products and have poor biocompatibility [22].
As discussed in this mini-review, the possibility to co-delivery multiple therapeutic agents and combine therapies offers a new dimension to this field of research. Not only hydrophilic/hydrophobic agents can be delivered at known concentration in time and space to reduce cancer cell growth, or angiogenesis [22]; also immunotherapies delivered by alginate-based hydrogels is an effective way to move forward and have more effective treatments [36,37]. Precision multi-functional alginate-based hydrogels could offer new strategies for the treatment of pathologies such as solid tumours, interdisciplinary research is required for a rapid clinical translation of the next generation alginate-based injectable systems.
Author Contributions: Conceptualization, A. T.; Investigation – X. B.; Writing - Original draft preparation, X. B.; Writing—Review and Editing, A. T.; Visualization, X. B. and A. T.; Supervision, A. T. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Data Availability Statement: In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. If the study did not report any data, you might add “Not applicable” here.
Acknowledgments: Xue Bai would like to thank Siriwat Sukphokkit for the scientific discussion during the writing of the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Bhattarai N.; Gunn J.; Zhang M.Q. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Delivery Rev., 2010, 62(1): 83-99. DOI: https://doi.org/10.1016/j.addr.2009.07.019
- Bai X.; Smith Z.L.; Wang Y.H.; et al. Sustained drug release from smart nanoparticles in cancer therapy: a comprehensive review. Micromachines, 2022, 13(10): 1623. DOI: https://doi.org/10.3390/mi13101623
- Anselmo A.C.; Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J. Controlled Release, 2014, 190: 15-28. DOI: https://doi.org/10.1016/j.jconrel.2014.03.053
- Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res., 2015, 6(2): 105-121. DOI: https://doi.org/10.1016/j.jare.2013.07.006
- Dimatteo R.; Darling N.J.; Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Delivery Rev., 2018, 127: 167-184. DOI: https://doi.org/10.1016/j.addr.2018.03.007
- Obara K.; Ishihara M.; Ozeki Y.; et al. Controlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in mice. J. Controlled Release, 2005, 110(1): 79-89. DOI: https://doi.org/10.1016/j.jconrel.2005.09.026
- Bos G.W.; Jacobs J.J.L.; Koten J.W.; et al. In situ crosslinked biodegradable hydrogels loaded with IL-2 are effective tools for local IL-2 therapy. Eur. J. Pharm. Sci., 2004, 21(4): 561-567. DOI: https://doi.org/10.1016/j.ejps.2003.12.007
- Thambi T.; Li Y.; Lee D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Controlled Release, 2017, 267: 57-66. DOI: https://doi.org/10.1016/j.jconrel.2017.08.006
- Clarivate. Web of science core collection. (accessed on 4 November 4 2022). https://clarivate.com/products/scientific-and-academic-research/research-discovery-and-workflow-solutions/web-of-science/web-of-science-core-collection/.
- Tanan W.; Panichpakdee J.; Saengsuwan S. Novel biodegradable hydrogel based on natural polymers: synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J., 2019, 112: 678-687. DOI: https://doi.org/10.1016/j.eurpolymj.2018.10.033
- Zhao W.; Jin X.; Cong Y.; et al. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol., 2013, 88(3): 327-339. DOI: https://doi.org/10.1002/jctb.3970
- Akindoyo J.O.; Mariatti M.; Hamid Z.A.A.; et al. Injectable hydrogel scaffold from natural biomaterials - An overview of recent studies. AIP Conf. Proc., 2020, 2267: 020068. DOI: https://doi.org/10.1063/5.0015786
- Lee K.Y.; Mooney D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci., 2012, 37(1): 106-126. DOI: https://doi.org/10.1016/j.progpolymsci.2011.06.003
- Bello A.B.; Kim D.; Kim D.; et al. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng., Part B, 2020, 26(2): 164-180. DOI: https://doi.org/10.1089/ten.teb.2019.0256
- Tang S.S.; Mohad V.; Gowda M.; et al. Insights into the role of collagen in vocal fold health and disease. Journal of Voice, 2017, 31(5): 520-527. DOI: https://doi.org/10.1016/j.jvoice.2017.01.008
- Bakshi P.S.; Selvakumar D.; Kadirvelu K.; et al. Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. Int. J. Biol. Macromol., 2020, 150: 1072-1083. DOI: https://doi.org/10.1016/j.ijbiomac.2019.10.113
- Abatangelo G.; Vindigni V.; Avruscio G.; et al. Hyaluronic acid: redefining its role. Cells, 2020, 9(7): 1743. DOI: https://doi.org/10.3390/cells9071743
- Aravamudhan A.; Ramos D.M.; Nada A.A.; et al. Chapter 4 — Natural polymers: polysaccharides and their derivatives for biomedical applications. Kumbar S.G.; Laurencin C.T.; Deng M. Natural and synthetic biomedical polymers. Oxfor: Elsevier, 2014: 67-89. DOI: https://doi.org/10.1016/B978-0-12-396983-5.00004-1
- Varghese S.A.; Rangappa S.M.; Siengchin S.; et al. Chapter 2 — Natural polymers and the hydrogels prepared from them. Chen Y. Hydrogels Based on Natural Polymers. Amsterdam: Elsevier, 2020: 17-47. DOI: https://doi.org/10.1016/B978-0-12-816421-1.00002-1
- Sun W.Z.; Gregory D.A.; Tomeh M.A.; et al. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci., 2021, 22(3): 1499. DOI: https://doi.org/10.3390/ijms22031499
- Tong Z.R.; Jin L.L.; Oliveira J.M.; et al. Adaptable hydrogel with reversible linkages for regenerative medicine: dynamic mechanical microenvironment for cells. Bioact. Mater., 2021, 6(5): 1375-1387. DOI: https://doi.org/10.1016/j.bioactmat.2020.10.029
- Fakhari A.; Subramony J.A. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Controlled Release, 2015, 220, Part A: 465-475. DOI: https://doi.org/10.1016/j.jconrel.2015.11.014
- Marques A.C.; Costa P.J.; Velho S.; et al. Stimuli-responsive hydrogels for intratumoral drug delivery. Drug Discovery Today, 2021, 26(10): 2397-2405. DOI: https://doi.org/10.1016/j.drudis.2021.04.012
- Ma Q.; Li Q.; Cai X.; et al. Injectable hydrogels as drug delivery platform for in-situ treatment of malignant tumor. J. Drug Delivery Sci. Technol., 2022, 76: 103817. DOI: https://doi.org/10.1016/j.jddst.2022.103817
- Zhao J.; Zhao X.; Guo B.L.; et al. Multifunctional interpenetrating polymer network hydrogels based on methacrylated alginate for the delivery of small molecule drugs and sustained release of protein. Biomacromolecules, 2014, 15(9): 3246-3252. DOI: https://doi.org/10.1021/bm5006257
- Ono K.; Saito Y.; Yura H.; et al. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res., 2000, 49(2): 289-295. DOI: https://doi.org/10.1002/(SICI)1097-4636(200002)49:2<289::AID-JBM18>3.0.CO;2-M
- Emoto S.; Yamaguchi H.; Kamei T.; et al. Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg. Today, 2014, 44(5): 919-926. DOI: https://doi.org/10.1007/s00595-013-0674-6
- Trombino S.; Servidio C.; Curcio F.; et al. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics, 2019, 11(8): 407. DOI: https://doi.org/10.3390/pharmaceutics11080407
- Andrade F.; Roca-Melendres M.M.; Durán-Lara E.F.; et al. Stimuli-responsive hydrogels for cancer treatment: the role of pH, light, ionic strength and magnetic field. Cancers, 2021, 13(5): 1164. DOI: https://doi.org/10.3390/cancers13051164
- Ruel-Gariépy E.; Shive M.; Bichara A.; et al. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm., 2004, 57(1): 53-63. DOI: https://doi.org/10.1016/S0939-6411(03)00095-X
- Qu J.; Zhao X.; Ma P.X.; et al. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater., 2017, 58: 168-180. DOI: https://doi.org/10.1016/j.actbio.2017.06.001
- Zhao L.L.; Zhu L.J.; Liu F.Y.; et al. pH triggered injectable amphiphilic hydrogel containing doxorubicin and paclitaxel. Int. J. Pharm., 2011, 410(1/2): 83-91. DOI: https://doi.org/10.1016/j.ijpharm.2011.03.034
- Iravani S.; Varma R.S. Alginate-based micro- and nanosystems for targeted cancer therapy. Mar. Drugs, 2022, 20(10): 598. DOI: https://doi.org/10.3390/md20100598
- Chao Y.; Liang C.; Tao H.Q.; et al. Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses. Sci. Adv., 2020, 6(10): eaaz4204. DOI: https://doi.org/10.1126/sciadv.aaz4204
- Ferreira N.N.; Ferreira L.M.B.; Miranda-Gonçalves V.; et al. Alginate hydrogel improves anti-angiogenic bevacizumab activity in cancer therapy. Eur. J. Pharm. Biopharm., 2017, 119: 271-282. DOI: https://doi.org/10.1016/j.ejpb.2017.06.028
- Alamzadeh Z.; Beik J.; Mirrahimi M.; et al. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur. J. Pharm. Sci., 2020, 145: 105235. DOI: https://doi.org/10.1016/j.ejps.2020.105235
- Xiao T.T.; Zhu J.Z.; Jia L.; et al. Injectable alginate hydrogels for synergistic tumor combination therapy through repolarization of tumor-associated macrophages. J. Controlled Release, 2022, 348: 239-249. DOI: https://doi.org/10.1016/j.jconrel.2022.05.049
- Mandal A.; Clegg J.R.; Anselmo A.C.; et al. Hydrogels in the clinic. Bioeng. Transl. Med., 2020, 5(2): e10158. DOI: https://doi.org/10.1002/btm2.10158





