
Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
Role of Sodium Glucose Cotransporter 2 Inhibitor in Hypertension
Zhitong Zhou, Daowen Wang, Junfang Wu *
Division of Cardiology, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei Key Laboratory of Genetics and Molecular Mechanisms of Cardiological Disorders, Wuhan 430030, China.
* Correspondence: Junfang.wu@tjh.tjmu.edu.cn
Received: 17 October 2022
Accepted: 15 November 2022
Published: 21 December 2022
Abstract: Sodium glucose cotransporter 2 inhibitors (SGLT-2i) are a new class of antidiabetic drugs that act by inhibiting the reabsorption of glucose in the proximal renal tubule, which results in lowering the level of blood and urinary glucose. Besides the glucose-lowing effect, some clinical trials found the benefits of SGLT2i in treating heart failure with or without diabetes. In 2021, SGLT2i were recommended by the European Society of Cardiology in treating of heart failure. Compared to heart failure, hypertension is a common cardiovascular disease with an increasing prevalence globally. There is also clinical evidence indicating that SGLT2i can lower blood pressure. Here we focused on addressing the role of SGLT-2i in treating hypertension and its possible mechanism in this review.
Keywords:
hypertension Sodium glucose cotransporter 2 inhibitor1. Introduction
Sodium glucose cotransporter 2 (SGLT2) inhibitors are a new kind of antidiabetic agent providing a unique insulin-independent glucose-lowering effect. Through inhibiting the SGLT2 in the proximal tubule (S1/S2 segments), the bulk of filtered glucose was excreted into the urine, thus achieving the goal of lowering the level of blood glucose [1]. Besides the glucose-lowing effect, a lot of large clinical randomized controlled trials in Type 2 Diabetes Mellitus (T2DM) patients also proved that SGLT2i reduced the rate of hospitalization for heart failure or kidney disease, diminished the risk of cardiovascular death, and delayed the progression of renal disease [2‒4]. Based on the outcomes of these trials, the American Diabetes Association (ADA) recommended SGLT2i as part of glucose-lowering therapy in T2DM patients with cardiovascular or renal disease. In 2019, a clinical trial of SGLT2i in patients having heart failure with reduced ejection fraction (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure, DAPA-HF) reported the benefits of SGLT2i in treating heart failure with the absence of diabetes for the first time [5]. Subsequently, some clinical trials confirmed its benefits in heart failure patients, including reduced and preserved heart failure (HFrEF, HFpEF) [6,7], which indicated its therapeutic potential in non-diabetes.
Compared to heart failure, hypertension is a common cardiovascular disease with an increasing prevalence worldwide. There is also some clinical evidence indicating that SGLT2i could lower blood pressure in diabetic patients with hypertension. In DECLARE‑TIMI 58 trial (Dapagliflozin Effect on CardiovascuLAR Events), significantly reduced systolic blood pressure and diastolic blood pressure were found in diabetic patients who received dapagliflozin treatment compared to subjects with placebo [4]. Similarly, a marked reduction in nighttime, daytime, and 24 hours systolic/diastolic blood pressure in older patients with diabetes and nocturnal hypertension was found in the group of dapagliflozin treatment [8]. Considering the blood pressure-decreasing effect of SGLT2i in diabetes with or without hypertension, here we mainly discuss the possibility of SGLT2i use in hypertension and its possible mechanisms in this review.
2. Reduced Myocardial Preload
Considering the mechanism of the hypoglycemic effect of SGLT2i and its osmotic diuretic effect subsequently, it is reasonable to postulate that decreasing blood volume caused by glycosuria (accompany by natriuresis) contributes to the antihypertension effect of SGLT2i (Figure 1). However, one clinical investigation found that empagliflozin exerts the same blood pressure lowering effect in chronic kidney disease (CKD) [9]. Theoretically, the lowering blood glucose effect and its subsequent decrease in blood volume may be weakened in CKD patients to some extent. So, these inconsistencies imply that there may other mechanisms to explain the lowering blood pressure effect of SGLT2i and support its application in treating hypertension patients without diabetes.
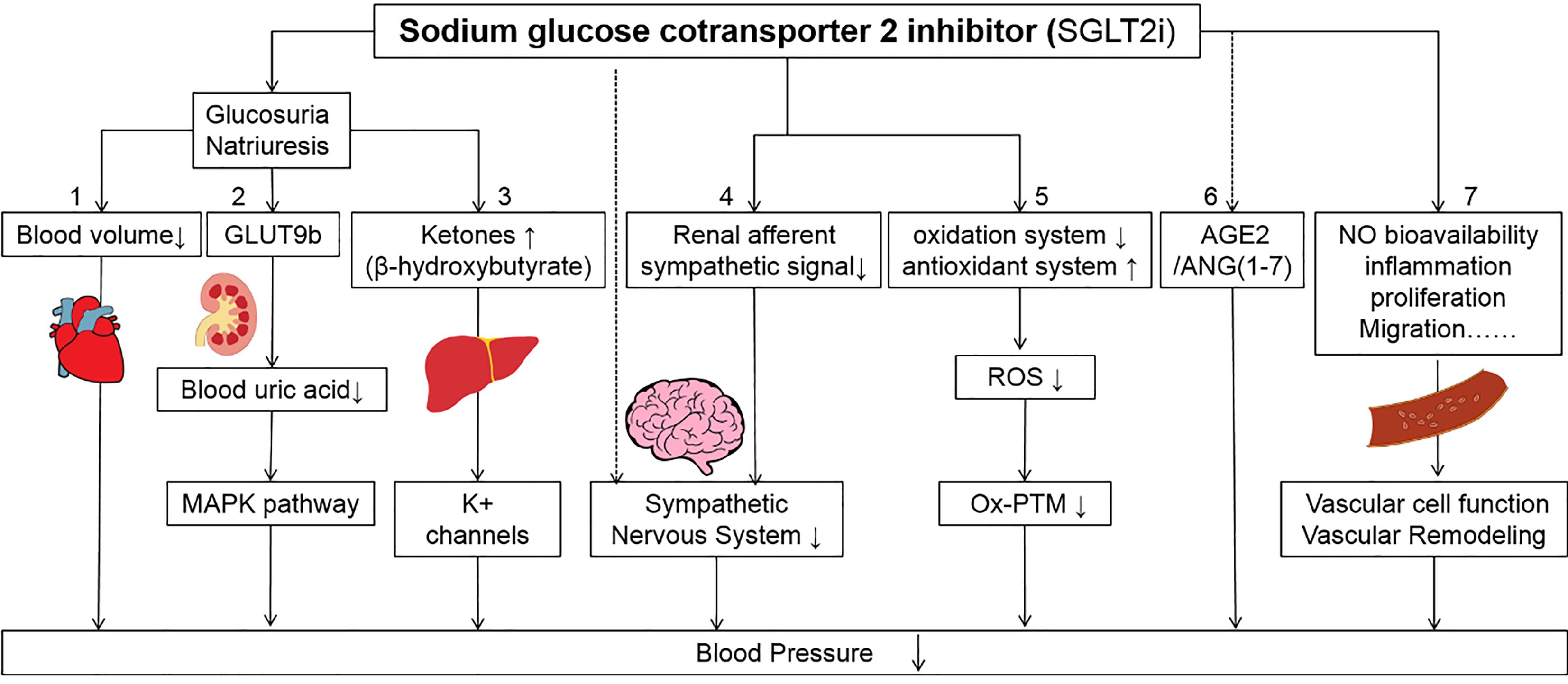
Figure 1. Suggested mechanisms of SGLT2i in lowering the blood pressure. (1) SGLT2i exerts its function of glucosuria (accompanying natriuresis) and decreases blood volume. (2) The increase of glucose in proximal convoluted tubule caused by SGLT2i competes with urate for GLUT9b (a kind of facilitative hexose/urate transporter) could increase the excretion of urate. Lowering the level of uric acid can prevent its adverse effects on blood pressure via the MAPK pathway. (3) SGLT2i can stimulate the production of ketones in liver. The increase of ketones may act on potassium channels directly that further cause vasodilation to lower blood pressure. (4) SGLT2i can reduce renal afferent sympathetic signal in the central nervous system to lower blood pressure. Besides, SGLT2i may directly act on some nuclei that express SGLT2 to reduce the activity of sympathetic nevous system. (5) SGLT2i can suppress the activity of oxidation system and stimulate the activity of antioxidant system which decrease the level of reactive oxygen species. (6) SGLT2i can act on the non-classic pathway to stimulate renin-angiotensin-aldosterone system (RAAS)-AGE2/ANG (1‒7) pathway when the classic ACE/ANG II is blocked. (7) SGLT2i can exert effects on the vascular function and remodeling directly or indirectly to lower blood pressure.
3. Metabolic Levels of Uric Acid or β-Hydroxybutyrate
A meta-analysis conducted by Abolfazl Akbari et al. evaluated the effect of different SGLT2is (canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, tofogliflozin, ertugliflozin, and luseogliflozin) on the level of serum uric acid in T2DM patients [10]. They found that all SGLT2is could reduce the level of serum uric acid compared with the placebo group [10]. The possible mechanism is that SGLT2i could suppress the activity of glucose transporter 9 (GLUT9b), a facilitative hexose/urate transporter mediating the reabsorption of filtered urate (another form of existence of uric acid in glomerular) in the first segment of the proximal convoluted tubule. When the function of SGLT2 is suppressed by SGLT2i, the concentration of glucose is increased in the proximal convoluted tubule and competes with urate for GLUT9b, hence increasing urinary uric acid level and lowering serum uric acid [10,11].
Hyperuricemia is considered to be involved in the pathogenesis of hypertension (Figure 1). The high level of uric acid could activate specific mitogen-activated protein kinases (MAPK)-like p38 and ERK44/42, and further activate the production of vascular renin‑angiotensin system (RAAS) components ‑angiotensinogen (AGT) and angiotensin II (AngII) or the expression of inflammatory mediators like C-reactive protein (CRP), by which affect proliferation and migration of vascular smooth muscle cell and vascular endothelial cell. Subsequently, the nitric oxide production of the vascular endothelial cells is inhibited and the oxidative stress of vascular smooth muscle cells is stimulated [12,13]. With the participation of these mechanisms, uric acid triggered vascular dysfunction and remodeling in the large and small arteries, which eventually resulted in hypertension [11]. For these reasons, the lowering uric acid effect may partially explain the lowering blood pressure ability of SGLT2i.
Besides the effects on blood glucose and serum uric acid, SGLT2i can increase the level of glucagon and subsequently reduce the level of insulin which mimics a state of fasting. Under such a state, the production of ketones increases which is generated by the liver used for energy supply [1,14]. Some experiments indicated that ketone bodies, like β-hydroxybutyrate, can be harnessed as a therapeutic strategy to combat hypertension (Figure 1). For example, Chakraborty et al. found the protective effects of β-hydroxybutyrate on salt-sensitive hypertension by inhibiting the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome in the kidney [15]. Besides, β-hydroxybutyrate can also act on potassium channels directly to further cause vasodilation [16], which partially explains the lowering blood pressure effect of SGLT2i.
4. Inhibited Over-Activation of Sympathetic Nervous System
Some clinical evidence supported that sympathetic neural factors are involved in the development and progression of hypertension [17]. For example, the activation of the sympathetic system can be observed in resistant hypertension, which is characterized by high blood pressure in spite of the continuous use of 3 or more antihypertensive drugs [17]. A clinical trial demonstrated that SGLT2i influences blood pressure in T2DM patients without an increase in heart rate [18], which indicated the effects of SGLT2i on the sympathetic nervous system. In a neurogenic hypertensive BPH/2J mice model, Herat et al found dapagliflozin significantly reduced the activity of the sympathetic nervous system, and resulted in significantly reduced tyrosine hydroxylase and norepinephrine levels in the kidney tissue [19], which supported the crosstalk between the sympathetic nervous system and SGLT2 regulation in hypertension.
There are several mechanisms to explain the influence of SGLT2i on the sympathetic nervous system (Figure 1). One possible mechanism is that SGLT2i reduces the renal afferent sympathetic signal to the central nervous system and further inhibits the over-activity of the sympathetic nervous system [1,20]. Other studies found that the use of dapagliflozin can influence the neuronal activity of several brain regions that express SGLT2, which implies the possibility of its direct effects on the central nervous system [21].
Keys: SGLT-2i, sodium glucose cotransporter 2 inhibitors; GLUT9b, Glucose Transporter 9; MAPK, mitogen-activated protein kinase; ROS, reactive oxygen species; Ox-PTM, oxidative posttranslational modifications; AGE, angiotensin-converting enzyme; ANG (1‒7), Angiotensin (1‒7). The ↓ means down-regulation; The ↑ means up-regulation.
5. Reduced Oxidative Stress
Many preclinical and clinical works found the involvement of oxidative stress in the development of hypertension [22,23]. For example, the level of oxidative stress biomarkers, such as glutathione peroxidase, malonyldialdehyde, and 4-hydroxynonenal were found to be positively correlated with the level of hypertension. Though oxidative posttranslational modifications (Ox-PTM) of some specific redox-sensitive related proteins, the reactive oxygen species (ROS) can provoke vascular dysfunction and remodeling, kidney injury etc., which further contribute to the development of hypertension [23].
The SGLT2i relieves the level of oxidative stress mainly through two aspects, diminishing the generation of free radicals and improving the ability of the antioxidant system (Figure 1). First, SGLT2i inhibits free radical production in multiple ways by suppressing the activity or expression of pro-oxidant enzymes, recovering mitochondrial dysfunction, lowering the production of advanced glycation end products (AGEs), suppressing the expression of inflammatory mediators in proximal tubular, correcting hemodynamic disturbances, insulin sensitivity and suppressing over activation of RAAS. On the other hand, SGLT2i can also increase or restore the expression of some anti-oxidases to exert its antioxidant effects [22]. Considering the balance between oxidative stress and hypertension, the anti-oxidative effects of SGLT2i may partially account for its antihypertension effects.
6. Regulation of the Renin-Angiotensin-Aldosterone-System (RAAS)
The RAAS is the main system to regulate hemodynamics in humans and its inadequate activation plays an important role in the development of hypertension [24]. In normal conditions, lower intrarenal pressure or lower concentration of sodium in macula densa cells stimulates the secretion of renin. The renin acts as a kind of enzyme that converts AGT to Angiotensin I (ANG I) which further converts to angiotensin II by angiotensin-converting enzyme (ACE) and plays a vasoactive role on blood vessels by constricting smooth muscle via Angiotensin II type 1 receptor (AT1R) [25,26]. Afterwards, angiotensin II stimulates aldosterone secretion which increases the reabsorption of sodium, whereas in the situation of increased concentration of sodium or high intrarenal pressure, there is an opposite process in RAAS to rectify such disturbed hemodynamics, which maintains the stability of hemodynamics. Besides, some works also found that ACE2/ANG (1‒7) axes were involved in the RAAS, which play a reverse role in vascular activity comparing classic ACE/ANG II [25,26].
The disorder of RAAS is considered to involve the development of hypertension. Although osmotic diuresis and plasma volume reduction caused by SGLT2i may influence RAAS by classic pathway theoretically, some animal and clinical studies indicated that the long term use of SGLT2i did not influence the level of plasmatic renin activity and concentration of aldosterone in T2DM, which reflect advantages of long-term application of SGLT2i in maintaining a stable level of classic RAAS components [27]. Some works also found that SGLT2i could activate the non-classic AGE2/ANG (1‒7) pathway in the context of a blockade on classic ACE/ANG II [26] (Figure 1). Such a possible mechanism of SGLT2i provides its potential function in treating hypertension, especially for those not susceptible to classic Angiotensin converting enzyme inhibitors (ACEI) /Angiotensin receptor blocker (ARB) treatment.
7. Vascular Function and Remodeling
SGLT2i also acts on vascular function and remodeling directly or indirectly. For vascular function, except for previous mechanisms like suppressing oxidative stress, improving the synthesis of NO, and further improving NO bioavailability, SGLT2i can also improve endothelium-dependent vasodilation in T2DM patients. Some clinical trials also found that SGLT2i can influence the proliferation and migration ability of vascular endothelial cells and smooth muscle cells (Figure 1). For vascular remodeling, SGLT2i can attenuate arterial stiffness and reduce the formation of the aortic aneurysm with the participation of multiple mechanisms like inhibiting inflammation, suppressing cell proliferation and migration etc., which are independent of its lowering blood glucose effects [28].
Since vascular dysfunction and remodeling are considered critical pathophysiological mechanisms involved in hypertension [29,30], the SGLT2i’s beneficial effects on vascular function and remodeling, imply its value in the treatment of hypertension patients without diabetes.
8. Future Perspectives
In this review, we described the lowering blood pressure effect of SGLT2i and its possible mechanism in treating hypertension. Through its influence on myocardial preload, metabolic level, sympathetic nervous system, oxidative stress, vascular function and remodeling, and RAAS, which is independent of its lowering glucose effect, SGLT2i may exert a lowering blood pressure effect in hypertension patients. Besides its protection of the heart and the kidney, there may be some special advantages in its use in hypertension patients, which can provide a therapeutic view for hypertension.
As for the possible mechanisms we describe above, these mechanisms do not work alone in the lowering blood pressure process of SGLT2i. There are interactions among them. For example, an increased level of uric acid could increase the RAAS activity and the production of inflammatory mediators, which further influence the proliferation and migration of vascular smooth muscle cells. The β-hydroxybutyrate can influence endothelium-dependent vasodilation which is an important aspect of vascular function involved in hypertension. The over-activation of the sympathetic system promotes the activity of RAAS, level of oxidative stress and vasoconstriction, vascular hypertrophy, and fibrosis, which further cause vascular dysfunction and remodeling. Besides, either RAAS or oxidative stress alone can play an important role in vascular dysfunction and remodeling [31], and their interactions between RAAS activity and the level of oxidative stress could not be ignored [32].
It's also notable that all these possible mechanisms of SGLT2i are not involved in any certain type of hypertension at the same time. For example, Zhao et al. found that Canagliflozin can improve vascular contraction and remodeling but not affect sympathetic nerve activity in Dahl salt-sensitive rats [33]. However, Lakshini et al. confirmed the inhibition effect on the sympathetic nervous system of dapagliflozin in BPH/2J mice [19]. Such controversial effects on sympathetic nerve activity may relate to different types of hypertension. Further animal or clinical trials would carry out to allow a deep understand the underlying mechanisms and confirm its rational use in different types of hypertension.
Before testing its efficiency, it is also necessary to confirm the long term safety of SGLT2i when using it as anti-hypertension drug. The good thing is that the SGLT2i has widely used in clinic. The adverse effects of SGLT2i in its application in diabetes are already reported, such as ketoacidosis, hypoglycaemia, genital or urinary tract infection, fracture and lower limb amputation [14,34]. The clinical application of SGLT2i in patients with heart failure also report some adverse effects like hypotension, genital and urinary tract infections etc.[5,6]. Considering above, it is necessary to evaluate safety of SGLT2i when using it to lower blood pressure.
Take these together, there may be several mechanisms involved in lowering the blood pressure effect of SGLT2i in hypertension, but which one is dominant and how they interact with each other are still unknown. Further preclinical and clinical studies need to be conducted for understanding the communication of these mechanisms of SGLT2i in terms of blood pressure reduction, as well as the possibility of SGLT2i application in hypertension in the clinic.
Author Contributions: Z.T.Z, D.W.W, and J.F.W have read and agreed to the published version of the manuscript.
Funding: This research was funded by National Key R&D Program of China (Grant No: 2022YFC3400700), National Natural Science Foundation of China (Grant No: 31971358, U22A20266) and Hubei Provincial Key Research and Developmental Program (Grant No: 2022BCA037).
Conflicts of Interest: The authors declare no conflict of interest.
References
- Vallon V.; Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu. Rev. Physiol., 2021, 83: 503-528. DOI: https://doi.org/10.1146/annurev-physiol-031620-095920
- Neal B.; Perkovic V.; Mahaffey K.W.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med., 2017, 377(7): 644-657. DOI: https://doi.org/10.1056/NEJMoa1611925
- Wanner C.; Inzucchi S.E.; Lachin J.M.; et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med., 2016, 375(4): 323-334. DOI: https://doi.org/10.1056/NEJMoa1515920
- Wiviott S.D.; Raz I.; Bonaca M.P.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med., 2019, 380(4): 347-357. DOI: https://doi.org/10.1056/NEJMoa1812389
- McMurray J.J.V.; Solomon S.D.; Inzucchi S.E.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med., 2019, 381(21): 1995-2008.
- Anker S.D.; Butler J.; Filippatos G.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med., 2021, 385(16): 1451-1461.
- Solomon S.D.; Vaduganathan M.; Claggett B.L.; et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC: Heart Failure, 2022, 10(3): 184-197.
- Kario K.; Okada K.; Kato M.; et al. 24-Hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation, 2018, 139(18): 2089-2097. DOI: https://doi.org/10.1161/CIRCULATIONAHA.118.037076
- Sternlicht H.; Bakris G.L. Blood pressure lowering and sodium-glucose co-transporter 2 (SGLT2is)inhibitors: more than osmotic diuresis. Curr. Hypertens. Rep., 2019, 21(2): 12. DOI: https://doi.org/10.1007/s11906-019-0920-4
- Akbari A.; Rafiee M.; Sathyapalan T.; et al. Impacts of sodium/glucose cotransporter-2 inhibitors on circulating uric acid concentrations: a systematic review and meta-analysis. J. Diabetes Res., 2022, 2022: 7520632. DOI: https://doi.org/10.1155/2022/7520632
- Bailey C.J. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes, Obes. Metab., 2019, 21(6): 1291-1298. DOI: https://doi.org/10.1111/dom.13670
- Corry D.B.; Eslami P.; Yamamoto K.; et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens., 2008, 26(2): 269-275. DOI: https://doi.org/10.1097/HJH.0b013e3282f240bf
- Kang D.H.; Park S.K.; Lee I.K.; et al. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol., 2005, 16(12): 3553-3562. DOI: https://doi.org/10.1681/ASN.2005050572
- Herrington W.G.; Savarese G.; Haynes R.; et al. Cardiac, renal, and metabolic effects of sodium-glucose co-transporter 2 inhibitors: a position paper from the European Society of Cardiology ad-hoc task force on sodium-glucose co-transporter 2 inhibitors. Eur. J. Heart Failure, 2021, 23(8): 1260-1275. DOI: https://doi.org/10.1002/ejhf.2286
- Chakraborty S.; Galla S.; Cheng X.; et al. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep., 2018, 25(3):677-689.e4. DOI: https://doi.org/10.1016/j.celrep.2018.09.058
- McCarthy C.G.; Chakraborty S.; Singh G.; et al. Ketone body β-hydroxybutyrate is an autophagy-dependent vasodilator. JCI insight, 2021, 6(20): e149037. DOI: https://doi.org/10.1172/jci.insight.149037
- Grassi G. The sympathetic nervous system in hypertension: roadmap update of a long journey. Am. J. Hypertens., 2021, 34(12): 1247-1254. DOI: https://doi.org/10.1093/ajh/hpab124
- Chilton R.; Tikkanen I.; Cannon C.P.; et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes, Obes. Metab., 2015, 17(12): 1180-1193. DOI: https://doi.org/10.1111/dom.12572
- Herat L.Y.; Magno A.L.; Rudnicka C.; et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC: Basic to Translational Science, 2019, 5(2): 169-179. DOI: https://doi.org/10.1016/j.jacbts.2019.11.007
- Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J. Cardiol., 2017, 71(5): 471-476. DOI: https://doi.org/10.1016/j.jjcc.2017.12.004
- Nguyen T.; Wen S.; Gong M.; et al. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting SGLT-2 in mice. Diabetes, Metab. Syndr. Obes.: Targets Ther., 2020, 13: 2781-2799. DOI: https://doi.org/10.2147/DMSO.S258593
- Yaribeygi H.; Atkin S.L.; Butler A.E.; et al. Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J. Cell. Physiol., 2019, 234(4): 3231-3237. DOI: https://doi.org/10.1002/jcp.26760
- Griendling K.K.; Camargo L.L.; Rios F.J.; et al. Oxidative stress and hypertension. Circ. Res., 2021, 128(7): 993-1020. DOI: https://doi.org/10.1161/CIRCRESAHA.121.318063
- Viola A.; Monticone S.; Burrello J.; et al. Renin and aldosterone measurements in the management of arterial hypertension. Horm. Metab. Res., 2015, 47(6): 418-426. DOI: https://doi.org/10.1055/s-0035-1548868
- Patel S.; Rauf A.; Khan H.; et al. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother., 2017, 94: 317-325. DOI: https://doi.org/10.1016/j.biopha.2017.07.091
- Puglisi S.; Rossini A.; Poli R.; et al. Effects of SGLT2 inhibitors and GLP-1 receptor agonists on renin-angiotensin-aldosterone system. Front. Endocrinol., 2021, 12: 738848. DOI: https://doi.org/10.3389/fendo.2021.738848
- Hou Y.C.; Zheng C.M.; Yen T.H.; et al. Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Int. J. Mol. Sci., 2020, 21(21): 7833. DOI: https://doi.org/10.3390/ijms21217833
- Durante W.; Behnammanesh G.; Peyton K.J. Effects of sodium-glucose co-transporter 2 inhibitors on vascular cell function and arterial remodeling. Int. J. Mol. Sci., 2021, 22(16): 8786. DOI: https://doi.org/10.3390/ijms22168786
- Prado A.F.; Batista R.I.M.; Tanus-Santos J.E.;et al. Matrix metalloproteinases and arterial hypertension: role of oxidative stress and nitric oxide in vascular functional and structural alterations. Biomolecules, 2021, 11(4): 585. DOI: https://doi.org/10.3390/biom11040585
- Endemann D.H.; Schiffrin E.L. Endothelial dysfunction. J. Am. Soc. Nephrol., 2004, 15(8): 1983-1992. DOI: https://doi.org/10.1097/01.ASN.0000132474.50966.DA
- Touyz R.M.; Alves-Lopes R.; Rios F.J.; et al. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res., 2018, 114(4): 529-539. DOI: https://doi.org/10.1093/cvr/cvy023
- Reina-Couto M.; Afonso J.; Carvalho J.; et al. Interrelationship between renin-angiotensin-aldosterone system and oxidative stress in chronic heart failure patients with or without renal impairment. Biomed. Pharmacother., 2021, 133: 110938. DOI: https://doi.org/10.1016/j.biopha.2020.110938
- Zhao Y.; Li L.; Lu Z.S.; et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin antagonizes salt-sensitive hypertension through modifying transient receptor potential channels 3 mediated vascular calcium handling. J. Am. Heart Assoc., 2022, 11(15): e025328. DOI: https://doi.org/10.1161/JAHA.121.025328
- Alqudsi M.; Velez J.C.Q.; Navarrete J. Medical management of resistant hypertension: the role of sodium-glucose cotransporter 2 inhibitors (SGLT2i). Current Opinion in Cardiology, 2021, 36(4): 420-428. DOI: https://doi.org/10.1097/HCO.0000000000000865





