Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
Recent Progress on Combustion Characteristics of Ammonia-Based Fuel Blends and Their Potential in Internal Combustion Engines
Denghao Zhu , and Bo Shu *
Department of Physical Chemistry, Physikalisch-Technische Bundesanstalt, Bundesallee 100, DE-38116 Braunschweig, Germany
* Correspondence: bo.shu@ptb.de
Received: 21 November 2022
Accepted: 22 December 2022
Published: 18 January 2023
Abstract: Ammonia has recently attracted numerous attentions from researchers and policy makers as promising energy and hydrogen carrier for mitigating the carbon footprints in the energy sector. The mature infrastructure for the production, storage, and transportation of ammonia allows a quick role of ammonia in energy systems. By applying green hydrogen and renewable energies, green ammonia can be produced, which makes ammonia even more attractive. Prior to the commercial use of ammonia for large-scale energy systems, e.g., internal combustion engines and stationary gas turbines, a fundamental understanding of the ignition and combustion behaviors of ammonia is essential. In the past decades, a lot of studies on ammonia combustion have been published. Those studies covered broad topics including experimental and numerical investigations which were either fundamental or practical oriented. To continuously follow state-of-the-art and to provide a brief overview of the most recent research on ammonia combustion so that others can easily identify the most relevant work, this review summarizes the recent progress on combustion characteristics of ammonia and ammonia fuel blends as well as their potential use in internal combustion engines. Combining the advantages and drawbacks identified in both fundamental and practical studies, a clearer road map for ammonia application is given.
Keywords:
ammonia ammonia-fuel blends combustion reaction kinetics internal combustion engine1. Introduction
Decarbonization in the energy and mobility sectors has been considered the most promising way for the mitigation of carbon dioxide (CO2) emissions that responses for climate change since the industrial revolution. To make power generation and mobility sustainable, two prerequisites should be fulfilled. First, the electricity used should be from renewable sources such as wind and solar energies, and second, if the electricity comes from power plants, carbon neutral or carbon zero energy carriers should be used. Considering both continuously increasing energy demands and fluctuations of renewable sources due to unpredictable weather changes, power generation applying chemical energy carriers must be applied still for a very long time before the renewable energies can fulfill the entire energy consumption. In the mobility sector, although short-range transportation such as passenger cars and trucks can be fully electrified, long-range transportation such as maritime and aviation applications will still rely on combustion-based propulsion.
Great efforts have been made to bring combustion-based power generation and propulsion to be independent of fossil sources [1,2]. The promising energy carriers can be characterized into two types, i.e., carbon neutral fuels and carbon zero fuels. Carbon neutral fuels have been introduced first as biofuels and then further developed along with the carbon capture, utilization and storage (CCUS) technology where the captured CO2 can be utilized for producing carbon-based fuels that are identical to the fossil fuel or having better characteristics by "tailored" processes via electro-chemistry, this application is also well-known as Power-to-X process and those fuels are also named as synthetic fuels [3]. Promising synthetic fuels are methanol, ethanol, oxymethylene ether etc. [4–6]. Carbon zero fuels contain no carbon atom in the molecule and so far are only hydrogen and ammonia. Hydrogen is a very promising future fuel that is a very reactive molecule owning promising energy content and can be easily produced in a water-splitting electrolyzer applying renewable energies; however, technical application of hydrogen still faces some obstacles such as high explosion risk and difficulties in storage and distribution [7,8].
The obstacles to hydrogen applications can be overcome by using ammonia as a hydrogen carrier. Ammonia is the most produced chemical on the planet. By applying green hydrogen and renewable power, green ammonia can be produced, i.e., hydrogen from water electrolysis and nitrogen separated from the air will be fed into the Haber process (also known as Haber-Bosch) that is powered by sustainable electricity. Owing to the well-established infrastructure, ammonia can be easily distributed and stored [9]. In comparison to hydrogen and nature gas, ammonia has a relative higher energy density (11.5 MJ/l) and it can be stored in liquid form under 10 bar at room temperature which makes ammonia a more attractive fuel. Due to this fact, fundamental combustion research on ammonia have been intensively conducted in the past few years [10–19]. The studies on neat ammonia combustion have reported that the ammonia has a very slow laminar burning velocity (LBV) and a very long ignition delay time (IDT), i.e., LBV = 0.06 m/s at equivalence ratio (ϕ) 1.05 at 1 bar and 295 K and IDT = 1383 ms at ϕ = 1.0 at 20 bar and 900 K [9]. Moreover, the flammability limit of ammonia is also very narrow, namely 15.5–27.0 vol.%. Given that ammonia is also a nitrogen-based molecule, the emissions of nitrogen oxide is also an open issue of its combustion.
Internal combustion engines (ICE) contribute over 99% of power on global transportation, in which 95% of global transport energy comes from petroleum-based fuels [20]. Reducing carbon emissions of ICEs is the core task to realize the decarbonization of the whole transportation system. By replacing fossil fuels with carbon-neutral or carbon-free fuels, ICEs can achieve zero environmental impact and continue to contribute as the power source. The potential of burning ammonia in ICEs has been explored in the past decades. The fact is that burning neat ammonia either in spark-ignition (SI) or compression-ignition (CI) engines is challenging due to its poor combustion properties. Combustion promoters such as hydrogen, alkanes and alcohols are required for better engine performance.
In a very recent review from Valera-Medina et al. [9] the potential of ammonia as fuel has been comprehensively studied where state of the art and the unsolved challenges of ammonia as the main energy vector have been reported. Since then, many works have been published reporting the new investigations on ammonia combustion including fundamental studies and practical applications. To the best knowledge of the authors, no further comprehensive review has been published for fundamental studies, i.e., reaction kinetics nor for internal combustion engines, namely automotive applications since then. Due to a large amount of literature, it is essential to conclude the current progress of ammonia combustion so that further research directions can be better addressed.
2. Fundamentals
A comprehensive understanding of the combustion chemistry of ammonia and ammonia-based fuel blends is the basis for their practical applications. To construct the chemistry from bottom up, fundamental studies including both experimental and theoretical investigations have been extensively reported in the past two years. The most investigated combustion parameters were laminar burning velocity, ignition delay time, and speciation. Compared to the summarized status in Valera-Medina et al. [9] many novel combustion promoters have been investigated, e.g., dimethyl ether, diethyl ether, alcohols etc. [21,22] and the combustion behavior under various conditions has been experimentally studied. Moreover, many new or updated chemical kinetics mechanisms have been reported for promising fuel blends [23–27]. Here, recent work within the past two years will be summarized in the following sections. It is worth to note only the studies taking ammonia as the main fuel, i.e., ammonia fraction was larger than 50% in fuel blends, are considered.
2.1. Laminar Burning Velocity
Laminar burning velocity (LBV), also known as laminar flame speed, is a key parameter for combustor design where the flammability, stability of the flame, flame propagation, and Makrstein length are the main experimental targets. In Valera-Medina et al. [9] the LBV of neat ammonia, ammonia/methane (CH4), ammonia/hydrogen (H2), ammonia/carbon moNOxide (CO), and ammonia/oxygen have been compared, it was clearly to see that oxygen has the most promoting effect and the order of enhancement of other promoters is H2 > CO > CH4. Due to various blending possibilities, more studies on LBV of ammonia enhanced by other combustion promoters have been performed and reported.
Shrestha et al. [28] extended the LBV measurement of ammonia/O2 mixtures in a constant volume combustion chamber (CVCC) under elevated temperature up to 373 K at 1 bar with oxygen content from 21% to 30% and the equivalence ratio (ϕ) varied between 0.8–1.4. It was found that the maximum LBV of ammonia/O2 blends increases as the oxygen content increases. The enhancement was assumed to be attributed to increased adiabatic flame temperature and the LBV shows quasi-linear dependence on the O2 content as the oxidizer. By comparing with the LBV of ammonia/H2 mixtures measured in the same work, a 9% increase of oxygen, i.e., 30% oxygen in total, has the same enhancement of adding 30% extra hydrogen in the fuel mixture which indicates rich oxygen combustion is a promising strategy. Besides normal fuel/oxygen/nitrogen mixtures, Shrestha et al. [28] also investigated the impact of diluent, namely, by replacing nitrogen with helium and almost doubled LBVs have been observed for the same ammonia/O2 mixtures under the same T, p, and ϕ. This observation again confirmed the impact of adiabatic flame temperature on the LBV. A complement to the study of Shrestha et al. [28], Vinod and Fang [29] investigated the LBV of ammonia/O2 mixture in a CVCC at both elevated T (373 K) and p (10 bar) covering a narrow ϕ between 0.9 and 1.15 with O2 content varying from 15% to 40%. The reported LBVs reach peak value at ϕ = 1.10 for almost all measured conditions and the maximum value obtained is this work is 112.7 cm/s at 40% O2 fraction. It is worth mentioning that the measured LBV of stoichiometric ammonia/O2 mixture by Vinod and Fang [29] at 21% O2, 373 K, and 10 bar is around 25 cm/s which is much higher than the measured LBV (7 cm/s) by Shrestha et al. [28] under the same temperature but at only 1 bar with a factor of 3.5. As the measured LBV in Shrestha et al. [28] agrees well with the other literature data at 1 bar and the LBV is also expected to be much lower at elevated pressure, the unusual data reported in Vinod and Fang [29] cannot be easily interpreted. Two possible explanations could be 1) the unit of the LBV reported in Vinod and Fang [29] should be mm/s instead of cm/s or 2) the ignition energy applied in Vinod and Fang [29] was 170 MJ which is too much higher than the normally required value (< 100 MJ) and therefore additional radical pool was built in the ignition process that highly enhanced the LBVs.
As literature studies demonstrated the promotion potential of methane [11,17], more studies were performed that extended the combustion enhancer to higher alkanes. Wang et al. [30] reported a comparative study on the LBV of C1-C4 alkane/ammonia blends in a CVCC at 298 K, 1 bar and ϕ = 1.0. It was observed that ethane/ammonia mixtures have the highest LBVs and ammonia content in fuel less than 20%. Higher LBVs were found in n-butane/ammonia blends at ammonia fraction in fuel >20%. By increasing ammonia mole fraction, reduction of LBV and increase of CO2 emissions have been observed. However, the behavior was found to be independent of the alkane types if the energy fraction of ammonia was used for the correlation. Markstein length (Lb) of the binary fuels has also been investigated by Wang et al. [30]. Lb of the alkane/ammonia mixtures increases first and then decreases with increasing ammonia fraction, and the Lb also increases with alkane size due to the increased effective Lewis number of the mixtures. Chen et al. [31] reported further a study on LBV of C1-C3/ammonia mixtures with and without enhancement by ozone (O3). The measurements were performed using Heat Flux (HF) method at 298 K, 1 bar, and covering ϕ from 0.6–1.6. The impact of O3 was studied by adding 5000 ppm O3 into the mixtures. The data measured by Chen et al. [31] at ϕ = 1.0 agree well with that of Wang et al. [30], indicating both measurements were reliable. Maximum LBV of C1-C3 alkane/ammonia was found at ϕ near 1.05 with and without the addition of O3. By looking into the LBVs in detail, enhancement of ozone is more significant at the leaner side rather than the rich side, e.g., more than 15% faster LBV was observed at ϕ = 0.6 and only 5% faster LBV was found at ϕ > 1.0. The ozone enhancement was found to be stronger in higher alkanes and the promotion effect of O3 was found to be attributed to O radical enrichment through early-stage O3 decomposition. Lavadera et al. [32] reported LBV measurements on binary fuel of ammonia and higher alkanes, namely, n-heptane and iso-octane applying HF method at 298 and 338 K under 1 bar and ϕ between 0.7 and 1.4. Here the LBV of ammonia/CH4 blends showed good agreement with literature data while discrepancy to literature data was found in fuel-rich ammonia/n-heptane and ammonia/iso-octane mixtures. A similar finding to Wang et al. [30] has been observed by Lavadera et al. [32] in binary ammonia/alkane fuels, i.e., the correlation between LBV and ammonia mass fraction in fuel blends is independent of alkane type. Moreover, as common recognition the enhancement of alkanes on LBVs is attributed to higher adiabatic flame temperature.
Besides simple molecules, oxygenates such as alcohols and ethers are also considered as the promoter for ammonia conversion as they have been well-recognized as combustion enhancers for conventional fossil fuels [33–35]. Issayev and co-works reported the pioneer studies on LBV of ammonia/dimethyl ether (DME) and ammonia/diethyl ether (DEE) mixtures [16,21]. The enhancement of DME on ammonia flame has been studied in a constant volume spherical reactor (CVSR) at 300 K, 1–5 bar, and ϕ = 0.8–1.3. The mole fraction of DME in fuel varies from 18% to 47% [21]. DME substantially enhanced the LBV of ammonia and LBV decreases as pressure increases. The highest LBV of ammonia/DME blends was found at ϕ = 1.1. The Markestein length Lb showed a monotonic decrease with ϕ for a DME ratio higher than 18% and showed an opposite trend correlated to the DEM blending ratio at the lean and rich sides of the mixtures. The Lb of fuel mixture with 18% DME showed similar behavior to neat ammonia, indicating the dominance of ammonia chemistry for this blend. Moreover, the cellular flame structure has been observed by increasing initial pressure and ammonia blending ratio. Xiao et al. [23] extended the LBV study on ammonia/DME mixtures with DME blending ratio up to 80% in fuel and with slightly wider ϕ, i.e., 0.7–1.5. The experimental observation was similar to that of Issayev et al. [21]. For ammonia/DEE mixtures, Issayev et al. [16] studied the LBV also in a CVSR at atmospheric pressure and temperature covering equivalence ratio between 0.9 and 1.3. The mole fraction of DEE in fuel varied from 10% to 40%. Similar to DME, DEE also enhances the LBV strongly and the enhancement efficiency of DEE is between H2 and CH4 and is higher than DME. More interestingly, the correlation between Lb and ϕ of ammonia/DEE mixtures was found to be reversed when DEE blending ratio increased indicates that dominating chemistry changed. As a follow-up, Shrestha and Issayev with co-workers [24], extended the investigation on LBV of ammonia/DEE blends to elevated pressures, i.e., 3 and 5 bar and the enhancement of DEE on LBV and suppress of pressure were further confirmed.
Wang et al. [22] reported a comprehensive study on the premixed ammonia/methanol and ammonia/ethanol flames using HF method at 1 bar and 298 to 448 K. The enhancement of ethanol on LBV of ammonia is higher than methanol. However, a general correlation has been identified; namely, the normalized LBV is proportional to the energy fraction of ammonia, in other words, this correlation is independent of the enhancers. This finding agrees with the aforementioned studies [16,32]. Pelé et al. [36] investigated the combustion behavior of ammonia/ethanol blends and the LBV was measured in a CVCC. A similar LBV-ϕ correlation has been observed in both Wang et al. [30] and Pelé et al. [36] but with inconsistent temperature dependence. Pelé et al. [36] claimed this discrepancy was due to the different facility effects between HF and CVCC methods.
Among such a number of new LBV studies, many authors tried to develop or update chemical kinetics mechanisms for reproducing their experimental data [21–24]. Along with the satisfied performance of those literature models, detailed modelling studies were performed to shed light on the chemistry. Despite the differences between the applied mechanisms, there were some interesting common findings:
(1) Carbon-nitrogen (C-N) interactions are less important for predicting LBVs under atmospheric conditions except for ammonia/methane blends,
(2) LBV are more sensitive to ammonia chemistry for ammonia/fuel blends at atmospheric pressures and C-N interaction becomes crucial at elevated pressures.
(3) NH2-hydrocarbon interaction and N2Hx chemistry need further investigation.
(4) The inhibition effect of ammonia in LBV is mostly attributed to radical scavenging.
(5) Enrichment of radical pool (O, H, OH) and higher adiabatic flame temperature is the key reason for higher LBV by applying combustion promoters.
To easily approach the relevant studies, an overview of the literature studies on LBV of ammonia/fuel blends in the years 2021 and 2022 can be found in Table 1. Moreover, the experimentally determined LBV of those blends are summarized in Figure 1, where the initial conditions are close to atmospheric temperature (298 K) and pressure (1 bar) having a volume fraction of the enhancer (DME, DEE, methanol, ethanol) in fuel 40% or 30% mole fraction in the total mixture (O2).
Table 1. List and detailed information of literature studies on laminar burning velocity (LBV) of ammonia/fuel blends.
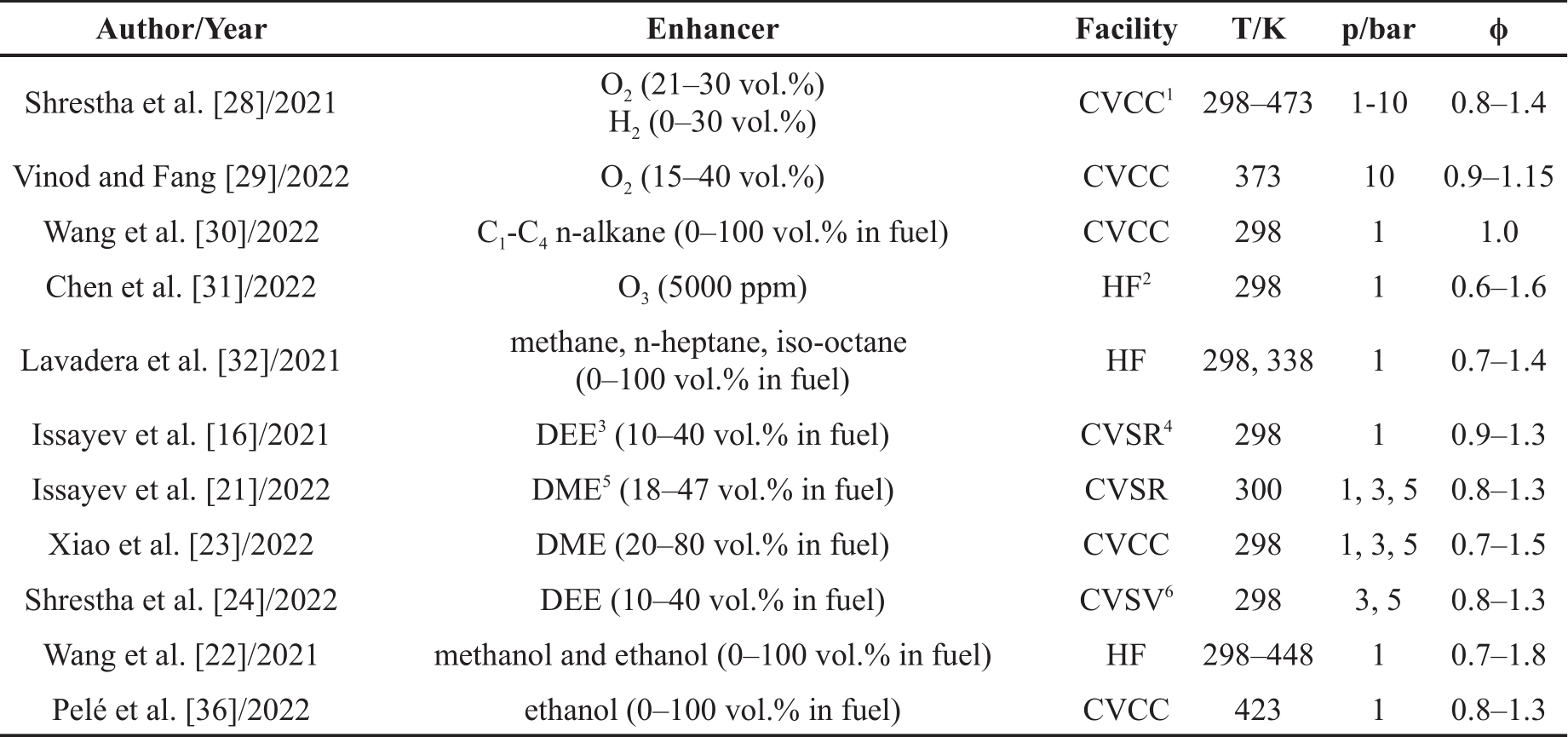
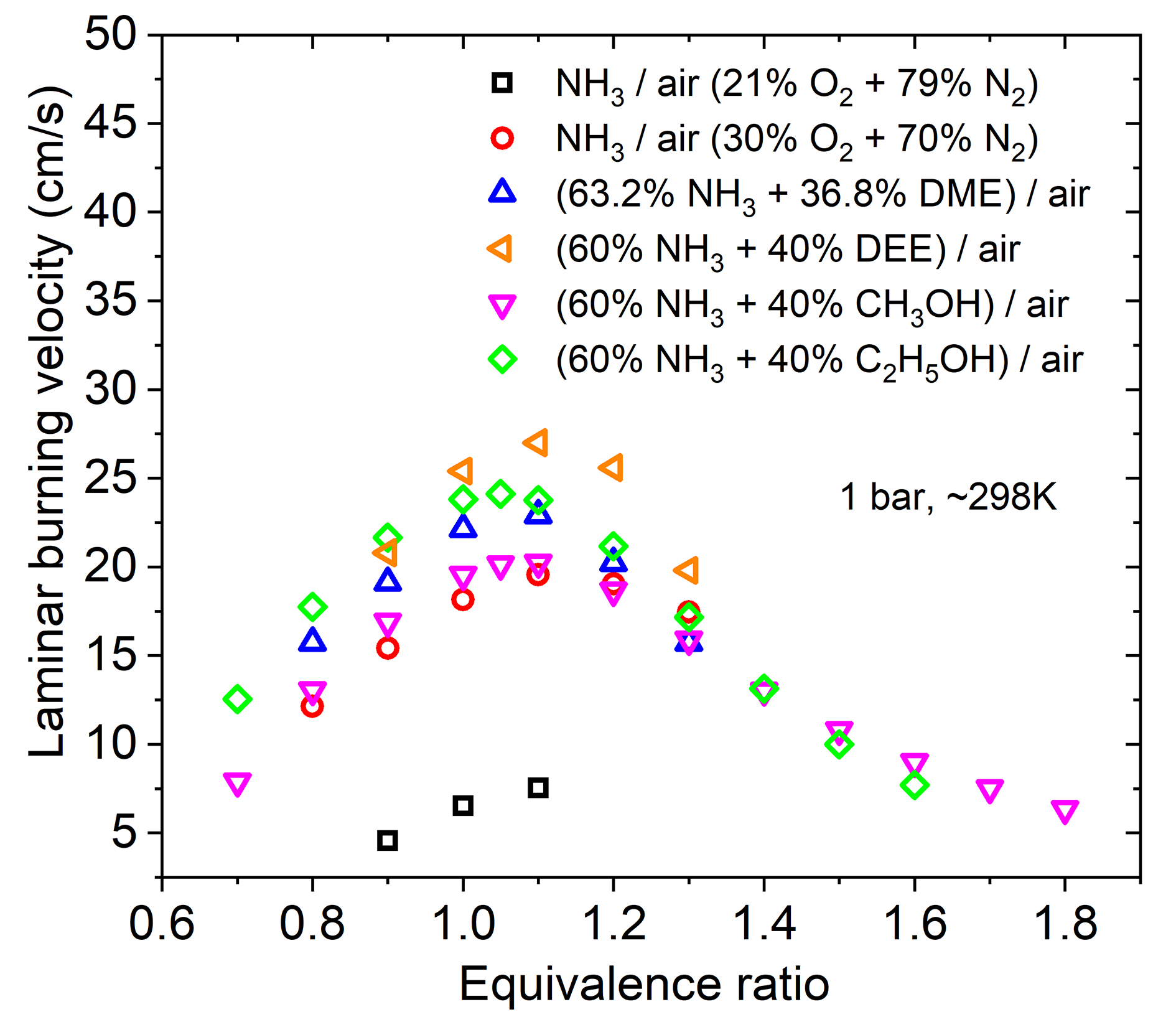
Figure 1. Comparison of the measured LBVs reported in literature applying O2 [28], dimethyl ether (DME) [21], diethyl ether (DEE) [16], methanol [22], and ethanol [22] as combustion enhancers.
2.2. Ignition Delay Time
Ignition delay time (IDT) is a key parameter for fuel characterizations. In fundamental studies, IDT is a popular validation target for testing the performance of developed chemical kinetics mechanisms [11–14]; for engine studies, IDT is normally applied to interpret abnormal combustion behaviors such as knocking in internal combustion engines [37–39]. Since the 1960s, IDT of ammonia has been experimentally investigated and a lot of new studies targeting ammonia and ammonia/fuel blends were reported in the past five years. In the review paper of Valera-Medina et al. [9] the most important IDT studies till the year 2020 have been well summarized. In the past two years, several more studies have been reported for investigating the IDT of ammonia/fuel blends which provide a wider range of data sets and new blending possibilities for engine-relevant conditions. Figure 2 depicts those experimental investigations on IDT of ammonia/fuel blends on a T-P diagram, where the covered and missing conditions can be directly found. Moreover, a list with detailed conditions of these studies can be found in Table 2.
Figure 2. Summary of the conditions in literature work on ignition delay time (IDT) measurements of ammonia/fuel blends.
Table 2. List and detailed conditions of literature work on ignition delay time (IDT) of ammonia/fuel mixtures.

As a complement to previous work, Chen et al. [40] studied the IDT of stoichiometric ammonia/H2 mixture in a shock tube that covered pressure of 0.12 and 10 bar at elevated temperatures which have never been reported before. There the H2 volume fraction in fuel varied between 0 to 70%. Chen and co-workers validated several literature mechanisms against the new data sets and found that the mechanism from Glarborg et al. [41] and Ottomo et al. [42] can well predict the IDTs. Similar to the findings reported in rapid compression machine (RCM) studies [43,44], the hydrogen chemistry starts to dominate as the blending ratio of H2 is higher than 5%. Dong et al. [45] performed further investigations on the IDT of stoichiometric ammonia/n-heptane mixtures at high temperatures (1000–1400 K) under 10 bar in a shock tube, which complements the data reported by Yu et al. [46] which were investigated in an RCM for low-temperature autoignition. It was observed that the effect of the ammonia blending ratio is smaller at high-temperature conditions than that at low temperatures. The modelling study via the newly developed mechanism showed that the H-abstraction from n-heptane by NH2 radicals is crucial for the accurate prediction of the IDTs.
Similar to LBV studies, several new studies focused on ammonia ignition enhanced by oxygenating fuels, i.e., dimethyl ether [21], methanol [25], and ethanol [26]. Issayev et al. [21] reported their new studies on ammonia/DME blends with DEM blending ration in fuel from 5 to 50%. RCM has been applied to interrogate the low-temperature combustion between 649 and 950 K under 20 and 40 bar, fuel-lean (ϕ = 0.5) and stoichiometric mixtures have been investigated. The measured IDTs with DME blending ratio 10% are similar to that of gasoline type FACE F but the negative temperature coefficient (NTC) effect of ammonia/DME is much weaker than FACE F. This implies a potential of ammonia/DME blends for direct utilization in current and future ICEs. Enhancement of DME on ammonia ignition was also found significant. 40% DME in fuel shows close IDT to neat DME indicating large ammonia blending ratio can be realized in practical applications, e.g., compression ignition engines. Li and co-workers reported two studies for ammonia/methanol [25] and ammonia/ethanol blends [26], respectively. Both studies were performed in an RCM and covered temperatures between 820–1120 K, pressure at 20 and 40 bar, and ϕ = 0.5, 1.0, and 2.0. Both methanol and ethanol demonstrate strong enhancement on the IDTs and the promotion efficiency of methanol and ethanol is much higher than H2 and CH4. Figure 3 left summarizes the measured IDT of ammonia/enhancer from the same research group [11,25,26,43] and the promoting effect of four fuel promoters can be easily concluded as ethanol > methanol > hydrogen > methane. Because the IDT of ammonia/DME and ammonia/DEE in the literature were measured by another research group [16,21], it is unable to put those experimental data in the same figure due to different mixture compositions and facility effects. However, as Issayev et al. [16,21] developed their own mechanisms for both fuel blends, simulated IDTs of seven ammonia-based fuels under the same initial condition, i.e., 40 bar, 570–1250 K, ϕ = 1.0, and blending ratio of enhancer = 5%, applying the corresponding best mechanisms [16,21,25,26] are plotted together in Figure 3 right, here the order of promoting effect is: DEE > DME > ethanol > methanol > hydrogen > methane.

Figure 3(a) comparison of the experimental IDTs of ammonia/fuel blends measured in the same group [11,25,26,43]; (b) comparison of simulated IDTs of ammonia/fuel blends under the same initial condition [16,21,25,26].
By applying IDTs as a validation target for mechanism development, it was commonly found that the carbon-nitrogen (C-N) cross reactions play a more important role than that for LBV simulations. Most difficulties in simulating IDTs were found by introducing a small amount of ignition promoters (1–5%), where the chemistry competition between N-based and C-based species is much stronger than fuel blends that have more than 20% enhancers where the chemistry of enhancers starts to dominate. Those effects can also be observed from the experimental data, e.g., the impact of equivalence ratio on IDTs is opposite for ammonia and for hydrocarbons. By mixing ammonia with hydrocarbon, there exists a certain blending ratio where the impact of ϕ on IDTs can be neglected due to the competing chemistry between ammonia and hydrocarbon.
Till now, there is no single literature mechanism that can reproduce all the experimental data including LBVs and IDTs of various blending strategies. It is a huge challenge to understand the combustion chemistry of ammonia, even if it is a simple molecule. To further support the model development, numerous new studies have been reported in the past few years [47–49] which provided huge data sets from speciation measurements for model refinement. Those data were measured in flames [47,48], flow reactors [49–52], jet stirred reactors [27,53–56], and shock tubes [57–62] via diagnostics such as molecular beam mass spectrometer (MBMS) [47,52,53,56], gas chromatography-mass spectrometer (GC/MS) [51,54,63], Fourier transform infrared spectrometer (FTIR) [27,55], tunable diode laser absorption spectroscopy (TDLAS) [57–62], and gas analyzers [49,51,55,63]. Temperature-resolved and time-resolved speciation data shed more light on the detailed reaction pathway and great improvement has been achieved from different groups. A comprehensive summary of the speciation studies is unfortunately out of the scope of this review. It is still worth mentioning that the N2Hx, HONO, HCNO, C-N interactions, NH3-NH2-NO recycling were identified as the most crucial sub-sets and further investigations (both experimental and theoretical) were strongly suggested by most researchers [47,51,59,62].
3. Internal Combustion Engines
The utilization of ammonia as an internal combustion engine fuel can be traced back to 1905 by Ammonia Casale, Ltd. [64]. As time goes by, the investigation of ammonia-fueled engines can be roughly divided into two stages with different motivations. In the middle of the twentieth century, ammonia gained attention as one of the alternative fuels because of the energy crisis. During World War II, the ammonia-fueled buses were equipped and put into service in Belgium to sustain its public transportation, against the background that diesel was not sufficiently available [64]. In the 1960s, Energy Depot Concept was proposed in the U.S., aiming to reduce the logistics problem of the military through synthesizing chemical fuels with air and water as the on-site raw materials [65]. Among all candidate alternative fuels, ammonia was considered the greatest potential regarding physical and chemical properties, handling, storage, and dispensing. Therefore, systematic investigations on ammonia in spark-ignition (SI) and compression-ignition (CI) engines were conducted in this era [66–70]. It was found that burning neat ammonia was possible, whereas a poor performance compared to neat gasoline or diesel engines. Many measures, including increasing ignition energy, multiple ignitions, increasing compression ratio, supercharging, installing swirl deflectors, enhancing intake temperature and pre-chamber ignition, were taken to improve the engine performance. A total of 45 gaseous and liquid additives were tested as combustion promoters, in which hydrogen and acetylene were found to be the two most promising combustion promoters for both SI and CI engines. Moreover, the compatibility study showed that ammonia and its combustion products with engineering materials and lubricants presented no substantial problem. In the following decades, the enthusiasm for studying ammonia-fueled engines declined with the lifting of the energy crisis [71]. Until recent ten years, ammonia is back in the spotlight as a sustainable energy carrier based on the consensus of reducing carbon emissions. Given the rapid development of engine technologies, such as accurate injection systems rather than the conventional carburetor, recent investigations conducted in modern engines are more informative for subsequent studies. Hence, the review below focuses on the recent progress on ammonia-fueled spark-ignition engines and ammonia-fueled compression-ignition engines, respectively.
3.1. Ammonia-Fueled Spark-Ignition Engines
Table 3 summaries the recent literature studies on the use of ammonia in spark-ignition engines. Most investigations chose hydrogen as a combustion promoter which will not cause additional carbon emission. Mørch et al. [72] conducted experiments with varying equivalence ratios, ammonia-to-hydrogen ratios and compression ratios on a CFR engine. Gaseous ammonia and hydrogen flow were controlled with two separate mass flow controllers (MFC) and introduced into a customized intake manifold. The results showed that a fuel mixture with 10 vol.% hydrogen performs best with respect to efficiency and power. The ammonia’s knock resistance allows higher thermal efficiency and power output than that of neat gasoline due to the possibility of a higher compression ratio. Lhuillier et al. [73–75] did a series of experiments using neat ammonia and ammonia/hydrogen fuel blends. The indicated mean effective pressure (IMEP) obtained with neat ammonia was comparable with those obtained with neat methane and ammonia/hydrogen mixtures. A maximal indicated thermal efficiency (ITE) close to 37% with a lean ammonia-air mixture was achieved, although high NOx emissions of up to 6000 ppm were measured. Increasing the intake pressure above 1.0 bar is a suitable strategy to promote neat ammonia combustion, which was similar to the previous attempt of using supercharging done by Cornelius et al. [66]. The addition of hydrogen is beneficial for very low cyclic variability, compensating for the shortfall of running neat ammonia at part-load conditions. Besides, with smaller than 10% hydrogen addition, the maximum ITE was enhanced to over 37%, mainly beneficial for the early stages of the combustion. The importance of the early combustion stage for ammonia combustion was also highlighted by the latest results conducted on an optical SI engine [76]. It should be mentioned that too high a hydrogen concentration can also result in deteriorating engine performance due to high heat loss.
Table 3. Recent literature studies of ammonia-fueled spark-ignition engines.

When using hydrogen as the combustion promoter, it can be either supplied from hydrogen cylinders or obtained directly from on-board ammonia dissociation. The latter seems a more promising method, given the difficulty in hydrogen storage. A numerical study on the effects of ammonia dissociating products on the combustion and emissions was performed by Zhang et al. [77]. The reaction mechanism used in the simulation was developed by Okafor et al. [78]. The composition of the reformate was set at a 2.4 volume ratio of hydrogen to nitrogen, assuming that the ammonia is completely consumed and the steam is removed by the intercooler. The optimal reformate blending ratio varies depending on the conditions. A moderate reformate blending ratio (less than 10%) can increase the ITE and power output while excessive reformate would cause negative work due to too early combustion phase. Mercier et al. [79] implemented such kind of assumption by using three separate cylinders of hydrogen, nitrogen and ammonia to simulate the situation of ammonia dissociation. It was confirmed that only a slight rate of ammonia dissociation (10%) considerably enhances the engine’s operating range thanks to better combustion stability. On-board dissociation of ammonia was realized by Frigo et al. [80,81] using a catalyst (RuCs/Al2O3). It was placed at the exit of the exhaust manifold to fully utilize the thermal energy of exhaust gases. The hydrogen flow rate supplied by the catalyst was proved to be larger than the minimum amount necessary for stable engine running. In a similar way, Ryu et al. [82] also installed an ammonia dissociation catalyst, although different catalytic materials with 2% ruthenium on 3.175 mm alumina pellets, in the engine exhaust line to obtain the necessary heat. The engine combustion was promoted and exhaust emissions were reduced after the catalyst was used. Koike et al. [83] did the first engine cold-start study in SI engines fueled with ammonia and its dissociation products. The initial investigation was conducted on a single-cylinder engine by using the cylinder to simulate the dissociation situation. Three ammonia adsorbers, H-ZSM-5, Cu-ZSM-5 and Pt-ZSM-5, were tested. Cu-ZSM-5 has the largest ammonia adsorption capacity, and favors lean burn operation for regeneration, while Pt-ZSM-5 has the advantage of simple regeneration control. Retarded ignition timing and high hydrogen content in the mixture were found to be necessary for the cold-start process to increase the exhaust gas temperature and reduce ammonia emissions. It took around 50 cycles to run steadily after the cold-start. During the cold-start process, with the help of the redox catalyst and adsorber, the ammonia emissions decreased from above 3000 ppm to almost zero. A further cold-start study was done by Koike et al. [84] on a four-cylinder engine equipped with an on-board ammonia reformer. An electrically heated ammonia-air mixture was provided to the reforming catalyst. Nearly zero ammonia emissions were achieved during cold start and fast idle until the engine warmed up by using Cu-ZSM-5 to adsorb unburned ammonia passing through a three-way catalyst before the catalyst was sufficiently warmed up.
The co-firing performance of ammonia-gasoline SI engines was also investigated since the whole system for gasoline is already there and minor changes need to be made. Grannell et al. [85] studied the optimal fuel mix map of ammonia-gasoline as a function of load, speed and compression ratio when utilizing gasoline as a combustion promoter. It shows that the requirement for gasoline goes down with increasing load and up with increasing speed. Due to a higher octane number of ammonia, the engine can operate at a much higher IMEP than that of neat gasoline. Besides, the ITE at moderate and high loads is better for ammonia with gasoline than for neat gasoline due to the better combustion phase. These results are consistent with the above-mentioned conclusions obtained by Mørch et al. [72]. As can be seen from Table 3, gaseous ammonia was mostly injected into the intake manifold or a mixing tank before entering the engine. Ryu et al. [86] did the first study utilizing direct injection of gaseous ammonia using a Parker Series 9 Pulse Valve injector and port-injection gasoline was used to promote the combustion. The injection pressure of ammonia is 13.79 bar at 40 °C. The advantage of direct injection of ammonia is higher volumetric efficiency. With this system, the engine power was increased to 2.7 kW which is much higher than the baseline power of 0.6 kW when using neat gasoline. All the above-mentioned injection strategies deal with gaseous ammonia. Haputhanthri et al. [87,88] studied the feasibility of using ammonia-gasoline liquid fuel blends with hermostated vapor liquid equilibrium high-pressure cells. Solubility test results prove that gasoline is capable of dissolving 23 g/L of ammonia on a mass basis at 3.45 bar pressure and 286.65 K temperature in a liquid phase. With the help of methanol and ethanol as emulsifiers, the solubility level can be increased. Gasoline with 10% methanol and ethanol can retain 68.4 g/L and 51.4 g/L of ammonia in the liquid phase, respectively. The engine performance does not alter using either ammonia-gasoline-methanol or ammonia-gasoline-ethanol fuel blends. Gasoline with 20% ethanol and 12.90% ammonia on a volume basis was identified as the optimum blend ratio in terms of engine power showing the positive impact of ammonia.
Apart from hydrogen and gasoline, there are also some studies about blending ammonia with other fuels in SI engines. Wu et al. [89] numerically studied the combustion of ammonia-methane fuel blends using the reduced chemical reaction mechanism developed by Li et al. [90]. The simulation time using the reduced mechanism can be reduced by a factor of 5 compared to the detailed mechanism with agreed predicted results. A 3D engine model was constructed where methane was injected additionally into the pre-chamber and resulting in a richer gas inside. The high-speed turbulent flame jets produced by the combustion of the rich mixture deeply travels into the main combustion chamber and ignites the lean mixture. Higher in-cylinder pressure and temperature were observed when more methane was added into the pre-chamber. However, the pre-ignition phenomenon may occur when excessive methane is added, leading to deteriorated combustion. Hence, the methane fraction range of 20–50% was recommended in this study. Oh et al. [91,92] experimentally investigated the feasibility of replacing partial natural gas by ammonia at different load conditions. Due to the lower flame speed of ammonia, ammonia addition resulted in lower brake power and indicated efficiency compared to the baseline. Conversely, if the baseline engine uses neat ammonia as the fuel, it means that adding natural gas can promote combustion. As mentioned above, the investigations by Haputhanthri et al. [87,88] indicate that ethanol can increase the solubility of ammonia in gasoline. This is caused by the polarities of ethanol and ammonia molecules providing a total solubility while gasoline molecules are not polarized. Given that, Pelé et al. [93] considered an efficient way would be only blending ammonia with ethanol. The liquid fuel blends were pressured with helium at 120 bar and directly injected into the combustion chamber. Two different injection strategies, namely homogenous and stratified modes, were studied. It was found that using neat ammonia was more restrictive to the injection strategy. To obtain homogeneous conditions, ammonia needs to be injected in advance to be fully premixed. In the stratified conditions, neat ammonia injection needs to be spitted with more than 50% of the fuel injected in homogeneous mode. These results indicate the enhancement in combustion stability after adding ethanol.
From the above discussions, although running neat ammonia in SI engines is possible, promoters are greatly beneficial in improving combustion stability and operating conditions. Hydrogen is the most widely tested promoter owing to its carbon-free characteristic. On-board dissociation of ammonia is a promising method for hydrogen generation to avoid bulk cylinders. Other promoters like gasoline, methane, natural gas and ethanol were also examined. A varying ammonia fraction in the fuel according to working conditions is always recommended regardless of the promoters used.
3.2. Ammonia-Fueled Compression-Ignition Engines
Burning neat ammonia in compression-ignition engines is even more challenging due to the low cetane number of ammonia and lacking stable ignition sources. As early as 1967, direct injection of neat liquid ammonia in a CI engine was attempted but failed even though the compression ratio was 30 [67]. Hence, combustion promoters are required for stable operation. The most used promoter in ammonia-fueled CI engines is diesel as shown in Table 4, where diesel is commonly used as a pilot fuel directly injected into the chamber to initiate the ignition of ammonia. Experimental results by Reiter et al. [94,95] demonstrated that ammonia could be successfully burned in a modern diesel engine with various speeds and loads. The energy replacement by ammonia can be as high as 95% for successful engine operation. Reasonable fuel economy can be obtained when ammonia provides 40-80% of the total energy. Niki et al. [96–100] conducted a series of work about the effect of injection strategies on ammonia-diesel CI engines. Advancing the injection timing of ammonia or diesel can reduce the NH3 and N2O emissions due to more homogeneous mixture whereas the HC and CO emissions are higher. The split diesel pilot injection is a method to resolve this above problem because of the reduction in the injected diesel fuel impingement to the combustion chamber wall. The benefit of the split diesel injection strategy was also confirmed by Yousefi et al. [101,102]. With the split diesel injection strategy, the ITE of the engine was increased up to 39.72%, which was higher than those obtained with a single injection strategy and neat diesel combustion. Besides, the greenhouse gas (GHG) emissions were reduced by 30.6% compared to that of the neat diesel case. Moreover, the unburned ammonia emissions were 83.5% less than the single injection strategy. Direct liquid ammonia injection, together with direct diesel injection using two separate injectors, was tried by Zhang et al. [103]. The high flexibility of this injection system can realize different combustion modes of ammonia, including premixed combustion, diffusion combustion and the premix-diffusion co-combustion. A clear two-stage combustion can be identified during the whole combustion duration, which is dominated by diesel and ammonia, respectively. A shorter combustion duration can be observed when running ammonia-diesel dual fuel modes compared to neat diesel cases.
Table 4. Recent literature studies of ammonia-fueled compression-ignition engines.
Numerical studies on ammonia-diesel CI engines have been conducted recently. Liu et al. [104] developed a skeletal ammonia/n-heptane mechanism with 104 species and 573 reactions to save the simulation time. The performance using gaseous or liquid ammonia ignited by a pilot diesel injected into the pre-chamber was compared. Both gaseous and liquid ammonia with pilot diesel is able to achieve a typical ship load range. Because of the higher swirl ratio, the liquid phase of ammonia has higher power output and thermal efficiency, which is consistent with the theoretical calculations by Starkman et al. [70]. Meanwhile, ammonia emissions are also lower using liquid ammonia owing to shorter injection duration and faster combustion rate. Another exploratory investigation on an ammonia-diesel stratified injection technology was also performed by Liu et al. [105]. This stratified injection was achieved by configuring different fuel injection rate-shape profiles of diesel and ammonia, respectively. Both ammonia and diesel were injected in the liquid phase from the same injector and there are three injectors in total. With this novel design, the engine can achieve similar power as a conventional diesel engine while greatly reducing CO2 and NOx emissions. The energy ratio of ammonia can be as high as 99% through the precise delivery of ammonia to the pilot fuel combustion zone. The NOx emissions can meet Tier III emission standards at 100% load without any after-treatment because of the heat absorption of ammonia vaporization. Li et al. [106] did the first high-pressure liquid ammonia spray study where the injection pressure was 600 bar and the temperature was maintained at 350 K. This data was then used to calibrate the spray model for engine simulation. A reaction mechanism consisting of 464 reactions and 76 species was developed through the combination of GRI3.0 and the n-heptane mechanisms. Two injection modes, namely high-pressure dual fuel (HPDF) and low-pressure dual fuel (LPDF), were numerically compared. For the HPDF mode, liquid ammonia and diesel were injected directly into the chamber with one injector. For the LPDF mode, the ammonia and air are fully mixed in the cylinder before diesel injection. The simulated results show that the maximum ammonia ratio of around 80% by energy is recommended for the LPDF mode, while the HPDF mode has the potential to achieve a 97% diesel replacement ratio. The LPDF mode has the potential to achieve a reduced cooling loss and therefore an increased indicated thermal efficiency, while the latter case can significantly reduce the engine-out NH3, NOx and GHG emissions.
Dissociated ammonia rather than neat ammonia co-firing with diesel in CI engines was also tested by several researchers. Gill et al. [107] compared the engine performance using neat ammonia, partially dissociated ammonia (1–2% NH3 + 75% H2 + 23–24% N2) and completely dissociated ammonia (100% H2, N2 removed by a diffusion membrane). The three mixture was simulated using bottled gasses. From an overall of view, the partially dissociated ammonia was the best way which has lower ammonia and significantly low N2O emissions compared to neat ammonia addition. Besides, the unburned hydrogen can be utilized in aftertreatment systems improving their activity at low temperatures. Using completely dissociated ammonia, namely pure hydrogen, was promising regarding combustion performance. However, the diffusion membrane requires high pressure and temperature to separate the hydrogen from the completely dissociated ammonia mixture, making it difficult and not practical to be implemented. On-board ammonia dissociation co-firing with diesel was realized by Kane et al. [108] through a thermochemical recuperation (TCR) reactor. The TCR consisted of a supported precious metal ammonia decomposition catalyst and a diesel oxidation catalyst (DOC) in the periphery. Unburned ammonia and hydrocarbon were oxidized by the DOC, resulting in additional heat from the exothermic reaction. Both the heat from the exhaust gas and the oxidation process in the DOC provided the heat for endothermic ammonia decomposition. At low speeds, the engine brake thermal efficiency (BTE) was slightly increased when dissociated ammonia was added, whereas deterioration occurred at high speeds. Therefore, a dynamic ammonia fuel fraction rather than a static replacement ratio was recommended to maximize its potential.
Considering the carbon-neutral property of biodiesel, replacing conventional diesel with biodiesel is promising for total carbon reduction. Reiter et al. [94] compared the performance of using ammonia-biodiesel and ammonia-diesel in CI engines and no big difference was found. The results from Nadimi et al. [109] confirmed the feasibility of burning ammonia-biodiesel in CI engines, which can achieve the same load as neat biodiesel with a slightly decrease in BTE. Elumalai et al. [110] did similar investigations but more positive results were obtained. Ammonia at 20%, 30%, 40% and 50% by the energy was injected in the manifold along with direct injection of algal biodiesel for achieving reactivity-controlled compression ignition (RCCI). An ammonia energy fraction of 44% was identified as the optimal value for RCCI mode. The BTE and brake specific energy consumptions were 35.13% and 10.84 MJ/kWh, respectively. Besides, HC, CO, CO2, NOx and smoke emissions were significantly reduced by 44%, 32%, 48%, 55%, and 66% when compared to neat biodiesel combustion.
Methoxymethane (DME) has a high cetane number and is a good candidate to initiate ammonia ignition. Similar to ethanol as mentioned above, DME and ammonia are miscible and the mixture can remain stable due to their polarities. Gross et al. [111] did the first study using a direct injection liquid ammonia-DME mixture in a CI engine. Tests were conducted using neat DME, 20 wt.% ammonia–80 wt.% DME mixture and 40 wt.% ammonia–60 wt.% DME mixture. After ammonia addition, the operating range of the engine was limited to medium load. To achieve stable combustion, the injection pressure needs to be increased from 150 bar to 200 bar and the intake temperature was raised from 30 °C to 80 °C when using 40 wt.% ammonia-60 wt.% DME mixture compared to neat DME. A double injection strategy was tried but it was unable to extend the engine operating range. Ryu et al. [112] extended the ammonia percentage in ammonia-DME mixture to a higher value (60 wt.% ammonia–40 wt.% DME). Significant cyclic variations were observed with such a high ammonia ratio, especially at low-load conditions. An appropriate injection timing ranging from 90 to 340° BTDC (before top dead center) can improve combustion stability.
The possibility of using ammonia in Homogeneous Charge Compression Ignition (HCCI) engines was discussed by Pochet et al. [113]. To stably run the engine under HCCI mode, the intake pressure was increased to 1.5 bar and the intake temperature was enhanced to 473 K with an effective compression ratio of 15.3 and an equivalence ratio of 0.28, achieving up to 70 vol.% ammonia proportion. The IMEP of pure hydrogen was limited to 2.7 bar restricted by knock and the load can be expanded to 3.1 bar after adding ammonia. However, the NOx emissions increased from 6 ppm of pure hydrogen to 1000–3500 ppm of ammonia-hydrogen. Pochet et al. [114] conducted further investigations on ammonia-hydrogen HCCI combustion on an even higher effective compression ratio of 22. The intake pressure was kept at 1 bar but the intake heating was still needed to ensure the desired temperature up to 513 K. A global 50% increase in IMEP compared to neat hydrogen was possible with 50 bar. A higher NOx emission of above 580 ppm was again observed after ammonia addition, against 50 ppm for neat hydrogen. Exhaust gas recirculation (EGR) can significantly reduce NO emission while increasing N2O emission due to lower temperature. Hence, boosted conditions with maximized stroke-to-bore ratios were suggested to allow higher EGR rates while maintaining combustion temperatures.
Running neat ammonia in CI engines is feasible, but many restrictions and effort, not only owing to the poor combustion properties of ammonia but also lacking stable ignition sources. High reactivity fuels like diesel and DME are required in CI engines, serving as the pilot fuel to initiate the ignition of ammonia. The injection strategy is essential to achieve reasonable engine performance.
3.3. Emissions
When considering launching ammonia-fueled vehicles on the road, emission problems should be carefully examined. The elements in fuel blends are decisive to the emission products. For neat ammonia or ammonia/hydrogen engines, the possible emission products include unburned ammonia, hydrogen and NOx (NO, NO2 and N2O). Hydrogen emission could also be detectable for neat ammonia that comes from the dissociation of ammonia. Unburned hydrocarbon, CO and soot (CI engines) are additional emissions when hydrocarbon exists in the fuel blends. Besides, CO2 emission is also discussed due to the carbon-free characteristic of ammonia, although generally CO2 is not considered as pollution emission. Considering the most complex case, eight kinds of emissions including NH3, NO, NO2, N2O, HC, CO, CO2 and soot need to be taken into consideration.
The emission of ammonia is a major concern due to its toxicity and a specific emission problem for ammonia-fueled engines. Generally, unburned ammonia emissions increase when higher ammonia in the fuel due to poor ignitability of ammonia and therefore, low combustion temperature. Flame quenching on the walls and ammonia trapped in the crevices also account for unburned ammonia emissions. The ammonia emission level in most literature studies is several thousand ppm, which greatly exceeds the ammonia slip emission limitation in Euro VI standard (0–10 ppm) as well as the human health regulations. Appropriate in-cylinder strategies and exhaust after-treatment methods are required to reduce unburned ammonia emissions. Ammonia adsorber such as Cu-ZSM-5 used by Koike et al. [83,84] could be an option which can achieve nearly zero ammonia emission at cold-start conditions.
NOx emissions are an unavoidable problem. The overall NOx emissions are governed by NO and N2O emissions.
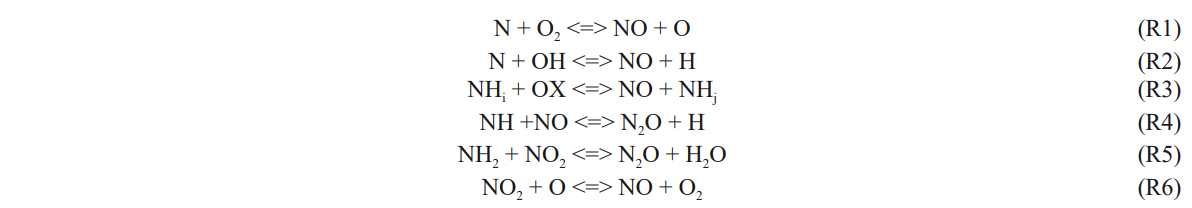
NO and NO2 participate in ozone layer depletion, cause respiratory problems and may be fatal under prolonged exposure. They are also acid rain precursors and can lead to photochemical smog. N2O is long-lived greenhouse gas of which the global warming potential is about 300 times that of CO2 and also responsible for ozone destruction. From the results of Westlye et al. [115], thermal NO (R1-R2) is dominated when the excess air ratio is below 1.2 both for the ammonia-hydrogen and gasoline engines. However, the second peak of NO appears only for the ammonia-hydrogen engine at a leaner mixture due to fuel-bound nitrogen (R3) at low temperatures. In Reaction 3, nitrogenous radicals (mainly NH and NH2) react with the O/H radical pool and O2 to generate NO. For the ammonia-hydrocarbon-fueled engine, NO emission is highly dependent on the ammonia content in the fuel. Both the results from Reiter et al. [94,95] and Yousefi et al. [101,102] showed that when ammonia energy fraction is less than 40%, NO emissions of ammonia-diesel dual-fuel operation are lower than those using neat diesel fuel. This could be partially caused by lower combustion temperature so that the thermal NO is reduced and partially attributed to the DeNOx process (NH/NH2 + NO). If ammonia energy fraction is further increased, although combustion temperature continues to decrease, the increase in fuel-bound nitrogen dominates. To reduce NO emission, a selected catalytic reduction (SCR) catalyst can be used since ammonia is already there. N2O is an intermediate species from ammonia reacting with NO (R4-R5) under certain conditions (1000 K to 1400 K). For hydrocarbon-fueled engines, the amount of N2O is negligible. However, the formation of N2O is one of the main concerns in ammonia combustion as a greenhouse gas. Generally, increasing ammonia fraction in the fuel increases N2O emissions, which would offset the benefit of lower CO2 emissions of using ammonia. According to the generation principle of N2O, increasing combustion temperature could be a feasible strategy to reduce N2O formation. Nonetheless, this might also promote the formation of NO. An explanation for the appearance of NO2 in combustion products from engines is that hot products with cooler gases cause Reaction 6 to quench. The share of NO2 in NOx is basically low, less than 4% in ammonia-hydrogen engines reported by Westlye et al. [115].
The emissions of HC and CO basically increase for higher ammonia fueling due to reduced flame temperature. While CO2 emissions significantly decrease due to the lack of chemical carbon in ammonia fuel. Note that when considering the whole GHG emissions, both N2O and CO2 emissions should be taken into consideration with different weights. As we all know, soot is produced from the rich zone of diesel combustion. When a significant amount of diesel is replaced by ammonia, fewer amounts of diesel fuel will be available to produce soot. Meantime, adding ammonia would reduce combustion temperature, and soot emissions might also be increased due to poor oxidation.
Overall, using ammonia as a fuel in the engine brings brand new emission problems, especially regarding unburned ammonia and N2O emissions. Trade-off relations exist between engine performance and emissions, as well as exist inside different emission sources. Combustion optimization strategies and appropriate after-treatment should be further investigated.
3.4. Discussions
According to the above literature reviews, the possible routes and key parameters for ammonia-fueled internal combustion engines are shown in Figure 4. Poor combustion characteristics of ammonia make it difficult to run neat ammonia in engines. Combustion promoters are required and different promoters have been studied. For SI engines, hydrogen is the most widely used promoter which can be obtained from cylinders or on-board ammonia dissociation. Few studies also discussed the potential of using gasoline, alkanes or alcohols as promoters in SI engines. Differently, the nature of compression-ignition requires high reactivity fuels as promoters like diesel and DME to initiate the ignition of ammonia. Key parameters highly related to engine performance should be well-optimized. For example, ignition timing generally should be advanced when using ammonia compared to hydrocarbon fuels because of lower burning velocity. Using high-performance ignition systems is also an option to improve combustion stability. Designing an appropriate injection system and finding the optimum injection strategies could be the most complex parts of ammonia-fuel engines. Even if burning neat ammonia, the phase of ammonia can be gaseous or liquid, and injection methods can be port injection or direct injection. When combustion promoters are introduced, the flexibility of injection increases and the complexity also increases significantly. Other parameters like compression ratio, intake charging or heating also have significant impacts on engine performance. Some studies have touched on parts of these patterns; nonetheless, the related researches are still scarce. Systematic investigations are strongly encouraged.
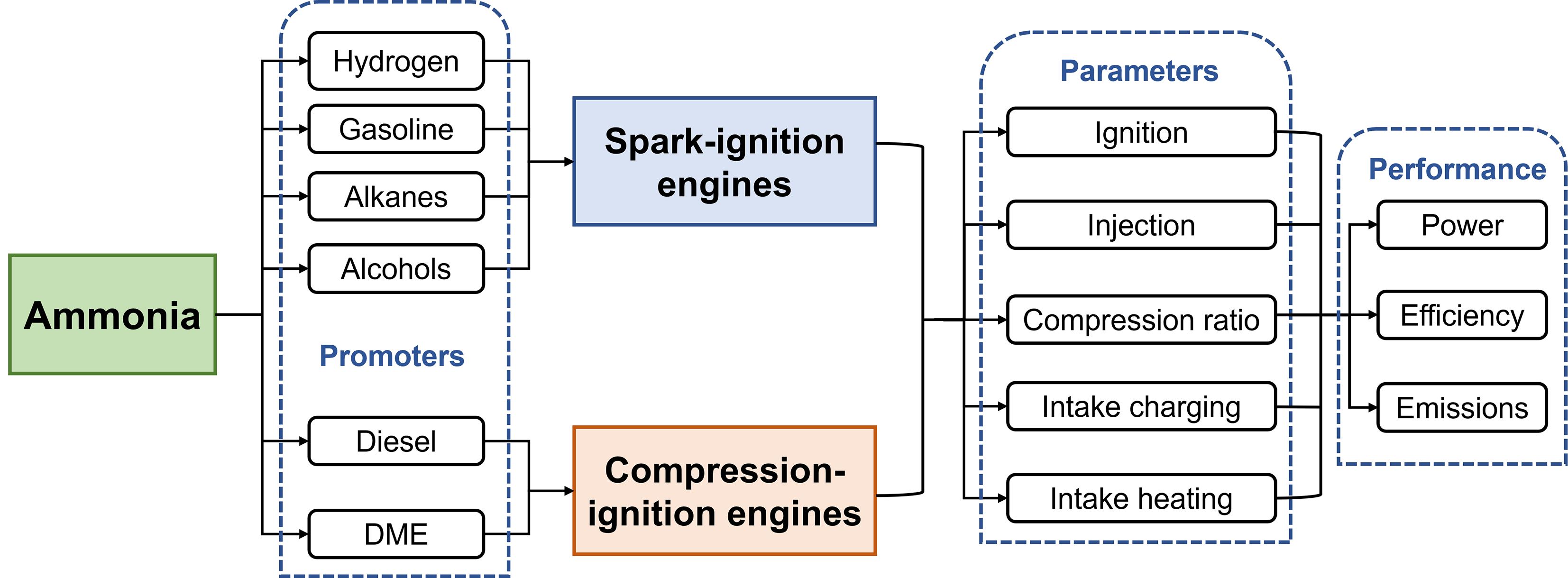
Figure 4. Possible routes and key parameters for ammonia-fueled internal combustion engines.
4. Conclusions and Outlook
Ammonia is attracting more attention in both the energy and mobility sectors where its application can mitigate carbon footprints immediately. To overcome the poor combustion properties of the ammonia blending strategy must be well studied before practical application. From the fundamental point of view, hydrocarbon enhancers are very promising for ammonia combustion and the most promising promoter found in this review is ethanol which has been well accepted in gasoline engines while the infrastructure for ethanol is the same mature as for ammonia.
Till now, fundamental studies on ammonia and ammonia/fuel blends have been very extensively performed and reported. The data sets of LBVs and IDTs are becoming larger and larger. However, blending ammonia with hydrocarbons becomes more attractive due to the fact that using ammonia can directly reduce CO2 emissions and the preferable combustion properties of hydrocarbons can be well-kept if a certain amount of hydrocarbon can be added as a fuel enhancer. Various blending strategies for different application scenarios make the research focus very diverse and there are still a lot of work that must be done to explore the combustion behavior of those fuel blends.
For understanding the combustion chemistry which is essential before new development of combustion systems, experimental and theoretical studies should be done to address missing reaction channels and to optimize rate constants or branching ratios of key elementary reactions, e.g., N2Hx, HNO, and H-abstraction via NH2. Although there are many experimental investigations on species determination, theoretical works involving high-level quantum chemistry calculations are still very scarce especially for the carbon-nitrogen cross-reactions. Carbon-nitrogen interactions have been identified as crucial for IDT predictions so that systematical investigations will benefit the entire community. Moreover, there were discrepancies between different studies for the same fuel system which are normally attributed to different facility effects. It is therefore essential to treat the experimental data with a proper uncertainty budget so that reliable evaluation of the model performance can be achieved.
The poor combustion properties of ammonia make it difficult or costly to run neat ammonia in internal combustion engines. From the literature review, hydrogen, which can be either packed in cylinders or obtained from on-board ammonia dissociation, is the most commonly used combustion promoter in ammonia-fueled spark-ignition engines. Some other promoters like gasoline, alkanes and alcohols were also tested. For ammonia-fueled compress-ignition engines, high-reactivity fuels like diesel and DME are required as pilot fuels to initiate the ignition of ammonia. Through co-firing strategies, combustion stability and engine performance can be significantly improved. Replacing fossil fuels with ammonia significantly reduces CO2 emissions (8–48% depending on different strategies). However, brand new problems also come out such as unburned ammonia and N2O emissions. A combination of optimum combustion strategies and specific after-treatment devices could be maximum compatible with power, efficiency and emissions. It should be mentioned that all studies were modified based on existing engines. Systematic investigations surrounding ammonia-fueled engines are still lacking, resulting in lots of open questions. From the author's point of view, it is necessary to develop dedicated engines for ammonia as it has totally different characteristics compared to widely used fossil fuels.
Author Contributions: Conceptualization, D. Z. and B. S.; formal analysis, D. Z. and B. S.; writing—original draft preparation, D.Z. and B.S.; supervision, B.S.; funding acquisition, D.Z. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding: Denghao Zhu would like to thank the Alexander von Humboldt Foundation for the financial support. The State of Lower Saxony/Germany for funding within the H2-WaVe project Grant number ZN3772 is grateful acknowledged by Bo Shu.
Data Availability: Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Kohse-HöinghausK.; OsswaldP.; CoolT.A.; et al. Biofuel combustion chemistry: from ethanol to biodiesel. Angewandte Chemie International Edition, 2010, 49(21): 3572–3597. DOI: https://doi.org/10.1002/anie.200905335
- ZhangC.; HuiX.; LinY.Z.; et al. Recent development in studies of alternative jet fuel combustion: progress, challenges, and opportunities. Renewable and Sustainable Energy Reviews, 2016, 54: 120–138. DOI: https://doi.org/10.1016/j.rser.2015.09.056
- IshaqH.; Crawford, C. CO2‑based alternative fuel production to support development of CO2 capture, utilization and storage. Fuel, 2023, 331, Part 2: 125684. DOI: https://doi.org/10.1016/j.fuel.2022.125684
- RasmussenP.B.; KazutaM.; ChorkendorffI. Synthesis of methanol from a mixture of H2 and CO2 on Cu(100). Surface Science, 1994, 318(3): 267–280. DOI: https://doi.org/10.1016/0039-6028(94)90101-5
- GongJ.L.; YueH.R.; ZhaoY.J.; et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. Journal of the American Chemical Society, 2012, 134(34): 13922–13925. DOI: https://doi.org/10.1021/ja3034153
- BaranowskiC.J.; BahmanpourA.M.; KröcherO. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): a review. Applied Catalysis B: Environmental, 2017, 217: 407–420. DOI: https://doi.org/10.1016/j.apcatb.2017.06.007
- Lubitz, W.; Tumas, W. Hydrogen: an overview. Chemical Reviews, 2007, 107(10): 3900–3903. DOI: https://doi.org/10.1021/cr050200z
- Dutta, review on productionS. A, storage of hydrogen and its utilization as an energy resource. Journal of Industrial and Engineering Chemistry, 2014, 20(4): 1148–1156. DOI: https://doi.org/10.1016/j.jiec.2013.07.037
- Valera-MedinaA.; Amer-HatemF.; AzadA.K.; et al. Review on ammonia as a potential fuel: from synthesis to economics. Energy & Fuels, 2021, 35(9): 6964–7029. DOI: https://doi.org/10.1021/acs.energyfuels.0c03685
- TakeyamaT.; MiyamaH. A shock-tube study of the ammonia-oxygen reaction. Symposium (International) on Combustion, 1967, 11(1): 845–852. DOI: https://doi.org/10.1016/S0082-0784(67)80210-8
- Shu, B.; He, X.; Ramos, C.F;et al. Experimental and modeling study on the auto-ignition properties of ammonia/methane mixtures at elevated pressures. Proceedings of the Combustion Institute, 2021, 38(1): 261–268. DOI: https://doi.org/10.1016/j.proci.2020.06.291
- PochetM.; DiasV.; MoreauB.; et al. Experimental and numerical study, under LTC conditions, of ammonia ignition delay with and without hydrogen addition. Proceedings of the Combustion Institute, 2019, 37(1): 621–629. DOI: https://doi.org/10.1016/j.proci.2018.05.138
- ShuB.; VallabhuniS.K.; HeX.; et al. A shock tube and modeling study on the autoignition properties of ammonia at intermediate temperatures. Proceedings of the Combustion Institute, 2019, 37(1): 205–211. DOI: https://doi.org/10.1016/j.proci.2018.07.074
- MathieuO.; PetersenE.L. Experimental and modeling study on the high-temperature oxidation of ammonia and related NOx chemistry. Combustion and Flame, 2015, 162(3): 554–570. DOI: https://doi.org/10.1016/j.combustflame.2014.08.022
- FengY.; ZhuJ.Z.; MaoY.B.; et al. Low-temperature auto-ignition characteristics of NH3/diesel binary fuel: ignition delay time measurement and kinetic analysis. Fuel, 2020, 281: 118761. DOI: https://doi.org/10.1016/j.fuel.2020.118761
- IssayevG.; GiriB.R.; ElbazA.M.; et al. Combustion behavior of ammonia blended with diethyl ether. Proceedings of the Combustion Institute, 2021, 38(1): 499–506. DOI: https://doi.org/10.1016/j.proci.2020.06.337
- DaiL.M.; GersenS.; GlarborgP.; et al. Autoignition studies of NH3/CH4 mixtures at high pressure. Combustion and Flame, 2020, 218: 19–26. DOI: https://doi.org/10.1016/j.combustflame.2020.04.020
- MeiB.W.; MaS.Y.; ZhangY.; et al. Exploration on laminar flame propagation of ammonia and syngas mixtures up to 10 atm. Combustion and Flame, 2020, 220: 368–377. DOI: https://doi.org/10.1016/j.combustflame.2020.07.011
- HanX.L.; WangZ.H.; CostaM.; et al. Experimental and kinetic modeling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air premixed flames. Combustion and Flame, 2019, 206: 214–226. DOI: https://doi.org/10.1016/j.combustflame.2019.05.003
- Kalghatgi, G. Is it the end of combustion and engine combustion research? Should it be? Transportation Engineering, 2022, 10: 100142. DOI: https://doi.org/10.1016/j.treng.2022.100142
- IssayevG.; GiriB.R.; ElbazA.M.; et al. Ignition delay time and laminar flame speed measurements of ammonia blended with dimethyl ether: a promising low carbon fuel blend. Renewable Energy, 2022, 181: 1353–1370. DOI: https://doi.org/10.1016/j.renene.2021.09.117
- WangZ.H.; HanX.L.; HeY.; et al. Experimental and kinetic study on the laminar burning velocities of NH3 mixing with CH3OH and C2H5OH in premixed flames. Combustion and Flame, 2021, 229: 111392. DOI: https://doi.org/10.1016/j.combustflame.2021.02.038
- XiaoH.H.; LiH.Z. Experimental and kinetic modeling study of the laminar burning velocity of NH3/DME/air premixed flames. Combustion and Flame, 2022, 245: 112372. DOI: https://doi.org/10.1016/j.combustflame.2022.112372
- ShresthaK.P.; GiriB.R.; ElbazA.M.; et al. A detailed chemical insights into the kinetics of diethyl ether enhancing ammonia combustion and the importance of NOx recycling mechanism. Fuel Communications, 2022, 10: 100051. DOI: https://doi.org/10.1016/j.jfueco.2022.100051
- LiM.D.; HeX.Y.; HashemiH.; et al. An experimental and modeling study on auto-ignition kinetics of ammonia/methanol mixtures at intermediate temperature and high pressure. Combustion and Flame, 2022, 242: 112160. DOI: https://doi.org/10.1016/j.combustflame.2022.112160
- LiM.D.; ZhuD.H.; HeX.Y.; et al. Experimental and kinetic modeling study on auto-ignition properties of ammonia/ethanol blends at intermediate temperatures and high pressures. Proceedings of the Combustion Institute, 2022, in press. DOI: https://doi.org/10.1016/j.proci.2022.07.151
- ZhangX.Y.; MoosakuttyS.P.; RajanR.P.; et al. Combustion chemistry of ammonia/hydrogen mixtures: jet-stirred reactor measurements and comprehensive kinetic modeling. Combustion and Flame, 2021, 234: 111653. DOI: https://doi.org/10.1016/j.combustflame.2021.111653
- ShresthaK.P.; LhuillierC.; BarbosaA.A.; et al. An experimental and modeling study of ammonia with enriched oxygen content and ammonia/hydrogen laminar flame speed at elevated pressure and temperature. Proceedings of the Combustion Institute, 2021, 38(2): 2163–2174. DOI: https://doi.org/10.1016/j.proci.2020.06.197
- VinodK.N.; FangT.G. Experimental characterization of spark ignited ammonia combustion under elevated oxygen concentrations. Proceedings of the Combustion Institute, 2022, in press.
- WangD.; WangZ.; ZhangT.Y.; et al. A comparative study on the laminar C1–C4 n-alkane/NH3 premixed flame. Fuel, 2022, 324, Part C: 124732. DOI: https://doi.org/10.1016/j.fuel.2022.124732
- ChenC.L.; WangZ.H.; YuZ.C.; et al. Experimental and kinetic modeling study of laminar burning velocity enhancement by ozone additive in NH3+O2+N2 and NH3+CH4/C2H6/C3H8+air flames. Proceedings of the Combustion Institute, 2022, in press. DOI: https://doi.org/10.1016/j.proci.2022.07.025
- LavaderaM.L.; HanX.L.; KonnovA.A. Comparative effect of ammonia addition on the laminar burning velocities of methane, n-heptane, and iso-octane. Energy & Fuels, 2020, 35(9): 7156–7168. DOI: https://doi.org/10.1021/acs.energyfuels.0c03424
- CinarC.; CanÖ.; SahinF.; et al. Effects of premixed diethyl ether (DEE) on combustion and exhaust emissions in a HCCI-DI diesel engine. Applied Thermal Engineering, 2010, 30(4): 360–365. DOI: https://doi.org/10.1016/j.applthermaleng.2009.09.016
- BaeC.; KimJ. Alternative fuels for internal combustion engines. Proceedings of the Combustion Institute, 2017, 36(3): 3389–3413. DOI: https://doi.org/10.1016/j.proci.2016.09.009
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Progress in Energy and Combustion Science, 2007, 33(3): 233–271. DOI: https://doi.org/10.1016/j.pecs.2006.08.003
- RonanP.; PierreB.; ChristineM.R.; et al. Laminar flame speed of ethanol/ammonia blends—an experimental and kinetic study. Fuel Communications, 2022, 10: 100052. DOI: https://doi.org/10.1016/j.jfueco.2022.100052
- SinghE.; ShankarV.B.; TripathiR.; et al. 2-Methylfuran: a bio-derived octane booster for spark-ignition engines. Fuel, 2018, 225: 349–357. DOI: https://doi.org/10.1016/j.fuel.2018.03.169
- JinS.Y.; ShuB.; HeX.Y.; et al. A study on autoignition characteristics of H2-O2 mixtures with diluents of Ar/N2 in rapid compression machine for argon power cycle engines. Fuel, 2021, 303: 121291. DOI: https://doi.org/10.1016/j.fuel.2021.121291
- BüttgenR.D.; RaffiusT.; GrünefeldG.; et al. High-speed imaging of the ignition of ethanol at engine relevant conditions in a rapid compression machine. Proceedings of the Combustion Institute, 2019, 37(2): 1471–1478. DOI: https://doi.org/10.1016/j.proci.2018.05.001
- ChenJ.D.; JiangX.; QinX.K.; et al. Effect of hydrogen blending on the high temperature auto-ignition of ammonia at elevated pressure. Fuel, 2021, 287: 119563. DOI: https://doi.org/10.1016/j.fuel.2020.119563
- GlarborgP.; MillerJ.A.; RuscicB.; et al. Modeling nitrogen chemistry in combustion. Progress in Energy and Combustion Science, 2018, 67: 31–68. DOI: https://doi.org/10.1016/j.pecs.2018.01.002
- OtomoJ.; KoshiM.; MitsumoriT.; et al. Chemical kinetic modeling of ammonia oxidation with improved reaction mechanism for ammonia/air and ammonia/hydrogen/air combustion. International Journal of Hydrogen Energy, 2018, 43(5): 3004–3014. DOI: https://doi.org/10.1016/j.ijhydene.2017.12.066
- HeX.; ShuB.; NascimentoD.; et al. Auto-ignition kinetics of ammonia and ammonia/hydrogen mixtures at intermediate temperatures and high pressures. Combustion and Flame, 2019, 206: 189–200. DOI: https://doi.org/10.1016/j.combustflame.2019.04.050
- DaiL.M.; GersenS.; GlarborgP.; et al. Experimental and numerical analysis of the autoignition behavior of NH3 and NH3/H2 mixtures at high pressure. Combustion and Flame, 2020, 215: 134–144. DOI: https://doi.org/10.1016/j.combustflame.2020.01.023
- DongS.J.; WangB.W.; JiangZ.Z.; et al. An experimental and kinetic modeling study of ammonia/n-heptane blends. Combustion and Flame, 2022, 246: 112428. DOI: https://doi.org/10.1016/j.combustflame.2022.112428
- YuL.; ZhouW.; FengY.; et al. The effect of ammonia addition on the low-temperature autoignition of n-heptane: an experimental and modeling study. Combustion and Flame, 2020, 217: 4–11. DOI: https://doi.org/10.1016/j.combustflame.2020.03.019
- TianZ.Y.; LiY.Y.; ZhangL.D.; et al. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combustion and Flame, 2009, 156(7): 1413–1426. DOI: https://doi.org/10.1016/j.combustflame.2009.03.005
- OsipovaK.N.; KorobeinichevO.P.; ShmakovA.G. Chemical structure and laminar burning velocity of atmospheric pressure premixed ammonia/hydrogen flames. International Journal of Hydrogen Energy, 2021, 46(80): 39942–39954. DOI: https://doi.org/10.1016/j.ijhydene.2021.09.188
- NakamuraH.; HasegawaS.; TezukaT. Kinetic modeling of ammonia/air weak flames in a micro flow reactor with a controlled temperature profile. Combustion and Flame, 2017, 185: 16–27. DOI: https://doi.org/10.1016/j.combustflame.2017.06.021
- MendiaraT.; GlarborgP. Ammonia chemistry in oxy-fuel combustion of methane. Combustion and Flame, 2009, 156(10): 1937–1949. DOI: https://doi.org/10.1016/j.combustflame.2009.07.006
- SongY.; HashemiH.; ChristensenJ.M.; et al. Ammonia oxidation at high pressure and intermediate temperatures. Fuel, 2016, 181: 358-–365. DOI: https://doi.org/10.1016/j.fuel.2016.04.100
- SunZ.J.; DengY.W.; SongS.B.; et al. Experimental and kinetic modeling study of the homogeneous chemistry of NH3 and NOx with CH4 at the diluted conditions. Combustion and Flame, 2022, 243: 112015. DOI: https://doi.org/10.1016/j.combustflame.2022.112015
- OsipovaK.N.; ZhangX.Y.; SarathyS.M.; et al. Ammonia and ammonia/hydrogen blends oxidation in a jet-stirred reactor: experimental and numerical study. Fuel, 2022, 310, Part A: 122202. DOI: https://doi.org/10.1016/j.fuel.2021.122202
- ArunthanayothinS.; StagniA.; SongY.; et al. Ammonia–methane interaction in jet-stirred and flow reactors: an experimental and kinetic modeling study. Proceedings of the Combustion Institute, 2021, 38(1): 345–353. DOI: https://doi.org/10.1016/j.proci.2020.07.061
- MannaM.V.; SabiaP.; SorrentinoG.; et al. New insight into NH3-H2 mutual inhibiting effects and dynamic regimes at low-intermediate temperatures. Combustion and Flame, 2022, 243: 111957. DOI: https://doi.org/10.1016/j.combustflame.2021.111957
- TangR.Y.; XuQ.; PanJ.Y.; et al. An experimental and modeling study of ammonia oxidation in a jet stirred reactor. Combustion and Flame, 2022, 240: 112007. DOI: https://doi.org/10.1016/j.combustflame.2022.112007
- AlturaifiS.A.; MathieuO.; PetersenE.L. An experimental and modeling study of ammonia pyrolysis. Combustion and Flame, 2022, 235: 111694. DOI: https://doi.org/10.1016/j.combustflame.2021.111694
- AlturaifiS.A.; MathieuO.; PetersenE.L. Shock-tube laser absorption measurements of N2O time histories during ammonia oxidation. Fuel Communications, 2022, 10: 100050. DOI: https://doi.org/10.1016/j.jfueco.2022.100050
- AlturaifiS.A.; MathieuO.; PetersenE.L. A shock-tube study of NH3 and NH3/H2 oxidation using laser absorption of NH3 and H2O. Proceedings of the Combustion Institute, 2022, in press. DOI: https://doi.org/10.1016/j.proci.2022.08.016
- HeD.; ZhengD.; DuY.J.; et al. Laser-absorption-spectroscopy-based temperature and NH3-concentration time-history measurements during the oxidation processes of the shock-heated reacting NH3/H2 mixtures. Combustion and Flame, 2022, 245: 112349. DOI: https://doi.org/10.1016/j.combustflame.2022.112349
- ZhengD.; HeD.; DuY.J.; et al. Shock tube study of the interaction between ammonia and nitric oxide at high temperatures using laser absorption spectroscopy. Proceedings of the Combustion Institute, 2022, in press. DOI: https://doi.org/10.1016/j.proci.2022.07.259
- ZhuD.H.; QuZ.C.; LiM.D.; et al. Investigation on the NO formation of ammonia oxidation in a shock tube applying tunable diode laser absorption spectroscopy. Combustion and Flame, 2022, 246: 112389. DOI: https://doi.org/10.1016/j.combustflame.2022.112389
- AriemmaG.B.; SorrentinoG.; RagucciR.; et al. Ammonia/methane combustion: stability and NOx emissions. Combustion and Flame, 2022, 241: 112071. DOI: https://doi.org/10.1016/j.combustflame.2022.112071
- Kroch, E. Ammonia-a fuel for motor buses. Journal of the Institute of Petroleum, 1945, 31: 213–223.
- Lindell, L. An introduction to the nuclear powered energy depot concept. New York: SAE International, 1965. DOI: https://doi.org/10.4271/650049
- CorneliusW.; HuellmantelL.; MitchellH. Ammonia as an engine fuel. New York: SAE International, 1965. DOI: https://doi.org/10.4271/650052
- Pearsall, T.J. Ammonia Application to Reciprocating Engines. Volume 1. CONTINENTAL AVIATION AND ENGINEERING CORP DETROIT MI. 1967.
- GrayD.S.; DomkeC.J.; MeguerianG.H.; et al. Ammonia application to reciprocating engines. Volume 2. Detroit MI, 1967.
- StarkmanE.S.; NewhallH.; SuttonR.; et al. Ammonia as a spark ignition engine fuel: theory and application. New York: SAE International, 1966. DOI: https://doi.org/10.4271/660155
- StarkmanE.S.; JamesG.E.; NewhallH.K. Ammonia as a diesel engine fuel: theory and application. New York: SAE International, 1967. DOI: https://doi.org/10.4271/670946
- BroK.; PedersenP.S. Alternative diesel engine fuels: an experimental investigation of methanol, ethanol, methane and ammonia in a D.I. diesel engine with pilot injection. New York: SAE International, 1977. DOI: https://doi.org/10.4271/770794
- MørchC. S.; BjerreA.; GøttrupM.P.; et al. Ammonia/hydrogen mixtures in an SI-engine: engine performance and analysis of a proposed fuel system. Fuel, 2011, 90(2): 854–864. DOI: https://doi.org/10.1016/j.fuel.2010.09.042
- LhuillierC.; BrequignyP.; ContinoF.; et al. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel, 2020, 269: 117448. DOI: https://doi.org/10.1016/j.fuel.2020.117448
- LhuillierC.; BrequignyP.; ContinoF.; et al. Combustion characteristics of ammonia in a modern spark-ignition engine. New York: SAE International, 2019. DOI: https://doi.org/10.4271/2019-24-0237
- LhuillierC.; BrequignyP.; ContinoF.; et al. Performance and emissions of an ammonia-fueled SI engine with hydrogen enrichment. New York: SAE International, 2019. DOI: https://doi.org/10.4271/2019-24-0137
- ZhangR.; ChenL.; WeiH.Q.; et al. Understanding the difference in combustion and flame propagation characteristics between ammonia and methane using an optical SI engine, Fuel, 2022, 324, Part C: 124794. DOI: https://doi.org/10.1016/j.fuel.2022.124794
- ZhangH.Y.Y.; LiG.S.; LongY.X.; et al. Numerical study on combustion and emission characteristics of a spark-ignition ammonia engine added with hydrogen-rich gas from exhaust-fuel reforming. Fuel, 2023, 332, Part 1: 125939. DOI: https://doi.org/10.1016/j.fuel.2022.125939
- OkaforE.C.; NaitoY.; ColsonS.; et al. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combustion and Flame, 2018, 187: 185–198. DOI: https://doi.org/10.1016/j.combustflame.2017.09.002
- MercierA.; Mounaïm-RousselleC.; BrequignyP.; et al. Improvement of SI engine combustion with ammonia as fuel: effect of ammonia dissociation prior to combustion. Fuel Communications, 2022, 11: 100058. DOI: https://doi.org/10.1016/j.jfueco.2022.100058
- FrigoS.; GentiliR. Analysis of the behaviour of a 4-stroke Si engine fuelled with ammonia and hydrogen. International Journal of Hydrogen Energy, 2013, 38(3): 1607–1615. DOI: https://doi.org/10.1016/j.ijhydene.2012.10.114
- FrigoS.; GentiliR.; de AngelisF. Further insight into the possibility to fuel a SI engine with ammonia plus hydrogen. New York: SAE International, 2014. DOI: https://doi.org/10.4271/2014-32-0082
- RyuK.; Zacharakis-JutzG.E.; KongS.C. Performance enhancement of ammonia-fueled engine by using dissociation catalyst for hydrogen generation. International Journal of Hydrogen Energy, 2014, 39(5): 2390–2398. DOI: https://doi.org/10.1016/j.ijhydene.2013.11.098
- KoikeM.; SuzuokiT. In-line adsorption system for reducing cold-start ammonia emissions from engines fueled with ammonia and hydrogen. International Journal of Hydrogen Energy, 2019, 44(60): 32271–32279. DOI: https://doi.org/10.1016/j.ijhydene.2019.10.105
- KoikeM.; SuzuokiT.; TakeuchiT.; et al. Cold-start performance of an ammonia-fueled spark ignition engine with an on-board fuel reformer. International Journal of Hydrogen Energy, 2021, 46(50): 25689–25698. DOI: https://doi.org/10.1016/j.ijhydene.2021.05.052
- GrannellS.M.; AssanisD.N.; GillespieD.E.; et al. Exhaust emissions from a stoichiometric, ammonia and gasoline dual fueled spark ignition engine. Proceedings of the ASME 2009 Internal Combustion Engine Division Spring Technical Conference, Milwaukee, Wisconsin, USA: ASME, 2009: 135–141. DOI: https://doi.org/10.1115/ICES2009-76131
- RyuK.; Zacharakis-JutzG.E.; KongS.C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Applied Energy, 2014, 116: 206–215. DOI: https://doi.org/10.1016/j.apenergy.2013.11.067
- Haputhanthri, S.O. Ammonia gasoline fuel blends: feasibility study of commercially available emulsifiers and effects on stability and engine performance. New York: SAE International, 2014. DOI: https://doi.org/10.4271/2014-01-2759
- HaputhanthriS.O.; MaxwellT.T.; FlemingJ.; et al. Ammonia and gasoline fuel blends for spark ignited internal combustion engines. Journal of Energy Resources Technology, 2015, 137(6): 062201. DOI: https://doi.org/10.1115/1.4030443
- WuX.F.; FengY.M.; XuG.D.; et al. Numerical investigations on charge motion and combustion of natural gas-enhanced ammonia in marine pre-chamber lean-burn engine with dual-fuel combustion system. International Journal of Hydrogen Energy, 2022, in press. DOI: https://doi.org/10.1016/j.ijhydene.2022.04.283
- LiR.; KonnovA.A.; HeG.Q.; et al. Chemical mechanism development and reduction for combustion of NH3/H2/CH4 mixtures. Fuel, 2019, 257: 116059. DOI: https://doi.org/10.1016/j.fuel.2019.116059
- OhS.; ParkC.; KimS.; et al. Natural gas–ammonia dual-fuel combustion in spark-ignited engine with various air–fuel ratios and split ratios of ammonia under part load condition. Fuel, 2021, 290: 120095. DOI: https://doi.org/10.1016/j.fuel.2020.120095
- OhS.; ParkC.; OhJ.; et al. Combustion, emissions, and performance of natural gas–ammonia dual-fuel spark-ignited engine at full-load condition. Energy, 2022, 258: 124837. DOI: https://doi.org/10.1016/j.energy.2022.124837
- PeléR.; BrequignyP.; BellettreJ.; et al. Performances and pollutant emissions of spark ignition engine using direct injection for blends of ethanol/ammonia and pure ammonia. THIESEL 2022 Conference on Thermo- and Fluid Dynamics of Clean propulsion Powerplants, Valencia, Spain: HAL, 2022: hal-03788532. DOI: https://doi.org/10.1177/14680874231170661
- ReiterA.J.; KongS.C. Demonstration of compression-ignition engine combustion using ammonia in reducing greenhouse gas emissions. Energy & Fuels, 2008, 22(5): 2963–2971. DOI: https://doi.org/10.1021/ef800140f
- ReiterA.J.; KongS.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel, 2011, 90(1): 87–97. DOI: https://doi.org/10.1016/j.fuel.2010.07.055
- NikiY.; YooD.H.; HirataK.; et al. Effects of ammonia gas mixed into intake air on combustion and emissions characteristics in diesel engine. Proceedings of the ASME 2016 Internal Combustion Engine Division Fall Technical Conference, Greenville, South Carolina, USA: ASME, 2016: V001T03A004. DOI: https://doi.org/10.1115/ICEF2016-9364
- NikiY.; NittaY.; SekiguchiH.; et al. Emission and combustion characteristics of diesel engine fumigated with ammonia. Proceedings of the ASME 2018 Internal Combustion Engine Division Fall Technical Conference, San Diego, California, USA: ASME, 2018: V001T03A016.
- NikiY.; NittaY.; SekiguchiH.; et al. Diesel fuel multiple injection effects on emission characteristics of diesel engine mixed ammonia gas into intake air. Journal of Engineering for Gas Turbines and Power, 2019, 141(6): 061020. DOI: https://doi.org/10.1115/1.4042507
- Niki, Y. Experimental investigation of effects of split diesel-pilot injection on emissions from ammonia-diesel dual fuel engine. Proceedings of the ASME 2021 Internal Combustion Engine Division Fall Technical Conference, Virtual, Online: ASME, 2021: V001T01A002. DOI: https://doi.org/10.1115/ICEF2021-66341
- Niki, Y. Reductions in unburned ammonia and nitrous oxide emissions from an ammonia-assisted diesel engine with early timing diesel pilot injection. Journal of Engineering for Gas Turbines and Power, 2021, 143(9): 091014. DOI: https://doi.org/10.1115/1.4051002
- YousefiA.; GuoH.S.; DevS.; et al. A study on split diesel injection on thermal efficiency and emissions of an ammonia/diesel dual-fuel engine. Fuel, 2022, 316: 123412. DOI: https://doi.org/10.1016/j.fuel.2022.123412
- YousefiA.; GuoH.S.; DevS.; et al. Effects of ammonia energy fraction and diesel injection timing on combustion and emissions of an ammonia/diesel dual-fuel engine. Fuel, 2022, 314: 122723. DOI: https://doi.org/10.1016/j.fuel.2021.122723
- ZhangZ.X.; LongW.Q.; DongP.; et al. Performance characteristics of a two-stroke low speed engine applying ammonia/diesel dual direct injection strategy. Fuel, 2023, 332, Part 2: 126086. DOI: https://doi.org/10.1016/j.fuel.2022.126086
- LiuL.; WuY.; WangY. Numerical investigation on the combustion and emission characteristics of ammonia in a low-speed two-stroke marine engine. Fuel, 2022, 314: 122727. DOI: https://doi.org/10.1016/j.fuel.2021.122727
- LiuL.; WuY.; WangY.; et al. Exploration of environmentally friendly marine power technology -ammonia/diesel stratified injection. Journal of Cleaner Production, 2022, 380, Part 1: 135014. DOI: https://doi.org/10.1016/j.jclepro.2022.135014
- LiT.; ZhouX.Y.; WangN.; et al. A comparison between low- and high-pressure injection dual-fuel modes of diesel-pilot-ignition ammonia combustion engines. Journal of the Energy Institute, 2022, 102: 362–373. DOI: https://doi.org/10.1016/j.joei.2022.04.009
- GillS.S.; ChathaG.S.; TsolakisA.; et al. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. International Journal of Hydrogen Energy, 2012, 37(7): 6074–6083. DOI: https://doi.org/10.1016/j.ijhydene.2011.12.137
- KaneS.P.; NorthropW.F. Thermochemical recuperation to enable efficient ammonia-diesel dual-fuel combustion in a compression ignition engine. Energies, 2021, 14(22): 7540. DOI: https://doi.org/10.3390/en14227540
- NadimiE.; PrzybyłaG.; EmbersonD.; et al. Effects of using ammonia as a primary fuel on engine performance and emissions in an ammonia/biodiesel dual-fuel CI engine. International Journal of Energy Research, 2022, 46(11): 15347–15361. DOI: https://doi.org/10.1002/er.8235
- ElumalaiR.; RaviK. Strategy to reduce carbon emissions by adopting ammonia–Algal biodiesel in RCCI engine and optimize the fuel concoction using RSM methodology. International Journal of Hydrogen Energy, 2022, 47(94): 39701–39718. DOI: https://doi.org/10.1016/j.ijhydene.2022.09.169
- GrossC.W.; KongS.C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel, 2013, 103: 1069–1079. DOI: https://doi.org/10.1016/j.fuel.2012.08.026
- RyuK.; Zacharakis-JutzG.E.; KongS.C. Performance characteristics of compression-ignition engine using high concentration of ammonia mixed with dimethyl ether. Applied Energy, 2014, 113: 488–499. DOI: https://doi.org/10.1016/j.apenergy.2013.07.065
- PochetM.; TruedssonI.; FoucherF.; et al. Ammonia-hydrogen blends in Homogeneous-Charge Compression-Ignition engine. New York: SAE International, 2017. DOI: https://doi.org/10.4271/2017-24-0087
- Pochet, M.; Jeanmart, H.; Contino, F. A 22:1 compression ratio ammonia-hydrogen HCCI engine: combustion, load, and emission performances. Frontiers in Mechanical Engineering, 2020, 6: 43. DOI: https://doi.org/10.3389/fmech.2020.00043
- WestlyeF.R.; IvarssonA.; SchrammJ. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel, 2013, 111: 239–247. DOI: https://doi.org/10.1016/j.fuel.2013.03.055






